Research Article
Cardiovascular Risk Factors and Adiponectin in Children and Adolescents with a Classic Congenital Adrenal Hyperplasia
3241
Views & Citations2241
Likes & Shares
Background: Congenital adrenal hyperplasia (CAH) is associated with hypocortisolism and hyperandrogenism, and when combined with glucocorticoid use, may cause predisposition to metabolic disorders.
Aim: To assess the cardiovascular risk factors in 25 children (19 girls) with CAH, and establish a correlation between glucocorticoid use and serum adiponectin levels.
Population and methods: In this cross-sectional study, we evaluated anthropometric parameters, systolic (SBP) and diastolic (DBP) blood pressure, stage of puberty, serum levels of glucose, insulin, adiponectin, androstenedione, lipid profile, and renin activity.
Results: Eleven (44%) patientswere overweight. In 12 (48%) patients, the waist circumference (WC) was ≥75th percentile for age and sex, and correlated with serum adiponectin levels and the body mass index z-score. Eight (32%) patients presented increased BP. SBP correlated with WC). One (4%) patient showed hyperinsulinemia and 18 (72%) showed an altered lipid profile, owing to a correlation between high-density lipoprotein (HDL) and androstenedione. Adiponectin levels were lower in the group treated with dexamethasone than those with prednisone/prednisolone and hydrocortisone (7.67 vs 12.34 vs 19.71 µg/mL, respectively).
Conclusion: High prevalence of obesity, increased BP, increased WC, and dyslipidemia were observed. WC positively correlated with SBP and negatively with adiponectin, whereas HDL was negatively associated with androstenedione.
INTRODUCTION
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders that cause a deficiency in the biosynthesis of adrenal cortisol [1,2]. About 90% to 95% of the cases are due to a mutation in the CYP21A2 gene, which encodes the 21-hydroxylase enzyme [2,3]. The clinical manifestations correlate to the degree of reduction of the enzymatic activity that allows the disease to vary from a classic form (salt-wasting and simple virilizing), the incidence which ranges from 1:10,000 to 1:15,000 live births, to a non-classic form with an incidence of 1:1,000 live births [4]. The result of this deregulation is a decreased production of cortisol and aldosterone, as well as an increase of precursors and pituitary secretion of adrenocorticotropic hormone, due to a negative feedback loop, which causes adrenal hyperplasia and enhanced androgen production [4,5]. In the classic form, prenatal hyperandrogenism leads to virilization of the external genitalia, causing genital ambiguity at birth in female infants [4]. Postnatal exposure to androgens results in the virilization, accelerated growth, advanced bone maturation, and early puberty [5-7]. The treatment consists of replacing, when necessary, glucocorticoids and mineralocorticoids. The corticosteroid of choice is hydrocortisone, administered at a dose of 10-15 mg/m-2/day-1. When hydrocortisone is not available, long-acting glucocorticoids such as prednisolone, prednisone, or dexamethasone could be used [4].
Despite the improvement in the treatment of this pathology through more effective corticosteroid-based therapies, the main limitation is to replicate the cortisol circadian rhythm, resulting in a difficult balance between hypercortisolism and hyperandrogenism [4-8].
Both hypercortisolism and hyperandrogenism have a deleterious effect on the metabolic control of the patients, predisposing them to develop risk factors for cardiovascular disease such as obesity, hyperinsulinemia with insulin resistance, dyslipidemia, and hypertension [9-15]. Moreover, the role of adipokines and their interference with the treatment of CAH patients are still to be elucidated [16].
Few studies in the literature evaluate the metabolic changes in children with CAH, and the most recent works show that factors such as obesity, dyslipidemia, hypertension, and insulin resistance are already present in childhood [9-15].
The aim of this study was to assess the presence of cardiovascular risk factors in children and adolescents with CAH, and establish a correlation with the glucocorticoids type used for treatment, disease control, and serum levels of adiponectin.
MATERIALS AND METHODS
Study Population and Sampling
In this cross-sectional study, 25 children and adolescents with a clear clinical and laboratory diagnosis of CAH (classic form), were under periodic and medical follow-up, and were not presenting other illnesses and/or acute or chronic associated metabolic changes were included.
Recruitment for inclusion in the study was carried out during the periodic check-up of the patients conducted at the Outpatient Clinic of Pediatric Endocrinology of the Instituto de Puericultura e Pediatria Martagão Gesteira, Federal University of Rio de Janeiro.
The study was approved by the ethics committee of the hospital, and all participants signed the free and informed consent form.
Data on age, sex, and type and dosages of glucocorticoids used in treatment were collected from medical records.
During physical examination, weight (child wearing only underwear), height (Tonelli stadiometer), stage of puberty, blood pressure, and waist circumference (WC) were measured.
The body mass index (BMI) was calculated using the following formula: weight (in kilograms) divided by height (in meters) squared. BMI and its standard deviation (SD) were assessed according to the sex and age of the patient by using the World Health Organization standards [17]. Obesity was considered to exist when the BMI percentile was ≥97 or SD ≥ +2 (in children older than 5 years), or with a BMI of ≥99.9th percentile or SD ≥ +3 (in children younger than 5 years). A child was considered as already being overweight with a BMI ≥ 85th percentile or SD ≥ +1 (in children older than 5 years), or a BMI ≥ 97th percentile or SD ≥ +2 (in children younger than 5 years) [17].
WC was measured in centimeters (cm) with the child standing, at the height just above the higher lateral edge of the right iliac crest, at the end of a normal exhalation, according to the recommendations of the National Health and Nutrition Examination Survey [18]. WC was considered to be increased when ≥75th percentile for age and sex [19].
Blood pressure (BP) was measured on the right arm, with the patient sitting at rest, using a sphygmomanometer and the appropriate cuff size according to the length of the patient’s arm. BP was expressed in percentile in agreement with the first Guideline on Prevention of Atherosclerosis in Childhood and Adolescence of the Brazilian Society of Cardiology [20]. The condition of the patient was considered normal when the percentiles of SBP and/or DBP were <90 mm Hg for height; prehypertension when the percentiles of SBP and/or DBP were >90 mm Hg and <95 mm Hg or whenever the BP was >120/80 mm Hg; stage 1 hypertension when the percentiles of SBP and/or DBP were between 95- and 99-mm Hg and increased by 5 mm Hg; stage 2 hypertension when the percentiles of SBP and/or DBP were >99 mm Hg and increased by 5 mm Hg. In patients 18 years old and above, BP was considered normal with an SBP and DBP of <130 and 85 mm Hg, respectively; borderline when SBP was between 130- and 139-mm Hg, and DBP between 85- and 89-mm Hg; stage 1 hypertension when SBP was between 140- and 159-mm Hg, and DBP between 90- and 99-mm Hg; and stage 2 hypertension when SBP was between 160- and 179-mm Hg, and DBP between 100- and 109-mm Hg [20]. Patients presenting an altered BP measurement were reevaluated on 3 different occasions to confirm the diagnosis.
The doses of glucocorticoids used for the treatment were converted into an equivalent dose of hydrocortisone, according to the following proportions: 1 mg of hydrocortisone was equal to 0.25 mg of prednisolone/prednisone and 0.037 mg of dexamethasone [21].
Laboratory Analyses
Blood samples were collected from fasting patients at 8 a.m. The serum levels of fasting glucose, total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were measured using Virto’s 5.1/FS, which calculates the concentrations through a dry chemistry method. Insulin and serum levels of androstenedione were assessed by chemiluminescence, whereas plasma renin activity was measured by radioimmunoassay. The levels of adiponectin were assessed by radioimmunoassay, in sera properly stored for this purpose, by using GAMA-C12 and kits based on a double-antibody polyethylene glycol technique, for which intra- and inter-assay coefficients of variation were between 1.7% and 6.2%, and between 6.9 and 9.2, respectively.
The index of homeostasis model assessment-insulin resistance (HOMA-IR) was calculated by multiplying the fasting glucose (mmol/L) by fasting insulin (µIU/mL) and dividing the result by 22.5 [22].
Statistical Analysis
Categorical variables were expressed as percentages or proportions. Pearson chi-square and Fisher's exact tests were used to compare the proportions.
Continuous variables were expressed as averages, medians, standard deviations, and interquartile distances. Student's t-test, analysis of variance (ANOVA), and Kruskal-Wallis test were used to compare the averages and medians, respectively.
Simple linear regression analysis was performed to assess the correlation between metabolic variables.
In all the statistical tests, a significance level of up to 5% (p ≤ 0.05) was adopted as a cutoff. The Epi Info 7.0 software was used for calculations.
RESULTS
General Characteristics
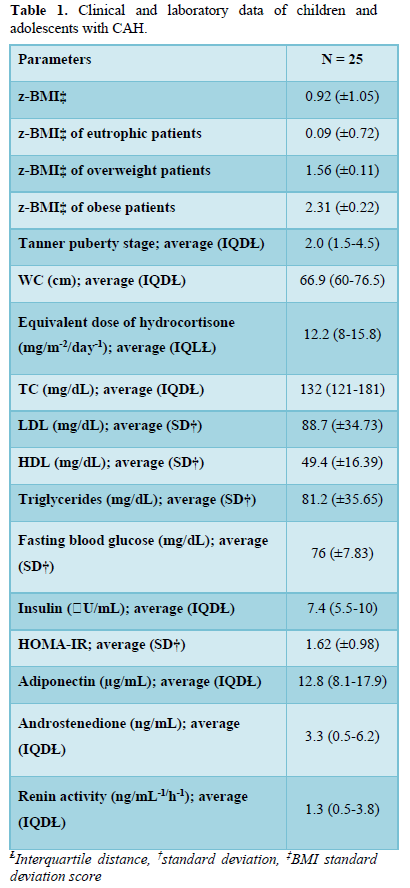
The age of the 25 study patients (19 girls and 6 boys) varied between 2 and 18.75 years, with an average age of 11.5 years and an interquartile distance (IQD) of between 8.0 and 13.4 years. A total of 13 patients were pubertal, 10 were prepubertal, and the remaining 2 were using a gonadotropin-releasing hormone (GnRH) analog to inhibit pubertal development. Concerning the clinical manifestations, 8 patients presented simple virilizing CAH and 17 were salt wasting. Concerning the treatment, 3 patients were receiving hydrocortisone, 14 prednisolone, 4 prednisone, and 3 dexamethasone, with an average equivalent dose of 12.2 mg/m-2/day-1 hydrocortisone (IQD: 8.0-15.8). Only 1 patient was not receiving glucocorticoids, owing to having terminated the treatment and returning to be re-monitored when the study started. A total of 17 salt-wasting patients were treated with fludrocortisone. Considering the laboratory control, 10 (40%) patients presented serum levels of androstenedione within the normal range, 14 (56%) patients displayed above-normal levels, and 1 patient (4%) had a level that was undetectable. The detailed clinical and laboratory data are shown in Table 1.
Nutritional Assessment
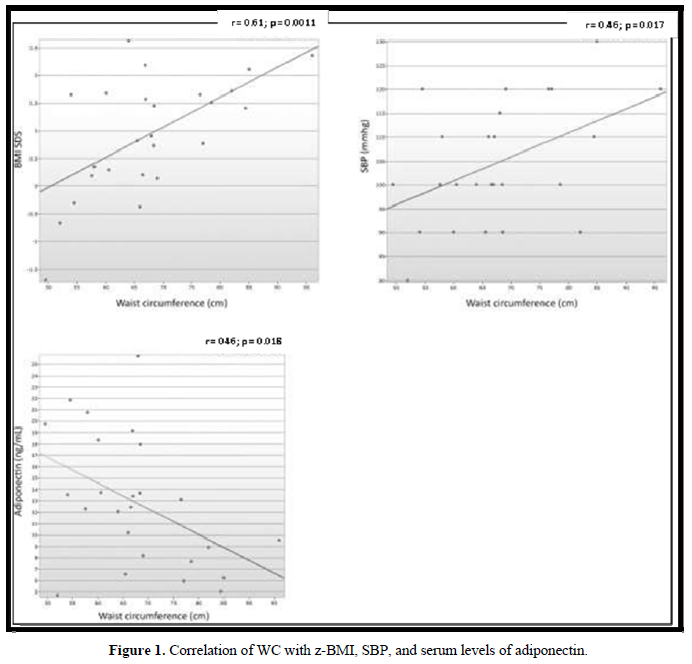
There were 13 patients (52%) who were eutrophic, 1 presented the risk of becoming overweight (4%), 7 (28%) were overweight, and 4 (16%) obese. Concerning WC, 12 patients (48%) presented a WC ≥75th percentile. No correlation was observed between the BMI z-score (z- BMI) and the chronological age, blood glucose, insulin, adiponectin, androstenedione, or the equivalent dose of hydrocortisone. A significantly positive correlation was detected between WC and z-BMI (r = 0.61; p = 0.0011) and SBP (r = 0.46; p = 0.017), and a negative correlation was observed with serum levels of adiponectin (r = - 0.46; p = 0.018) (Figure 1).
Blood Pressure
A total of 17 (68%) patients were normotensive, 6 (24%) were prehypertensive, and 2 (8%) presented stage 1 hypertension. Among the patients with altered BP, 2 presented only an increase in SBP, 4 only an increase in DBP, and 2 an increase in both SBP and DBP. No correlation was observed between the values of SBP or DBP and the equivalent dose of hydrocortisone, fludrocortisone, z-BMI, serum levels of adiponectin, and renin activity.
Lipid Profile, Insulin, and Adiponectin
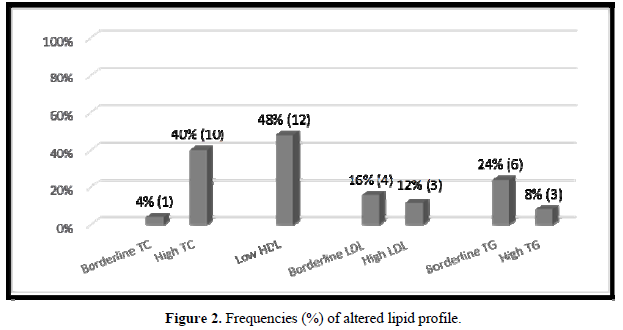
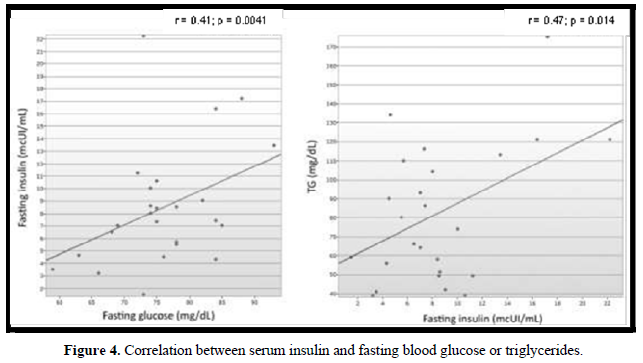
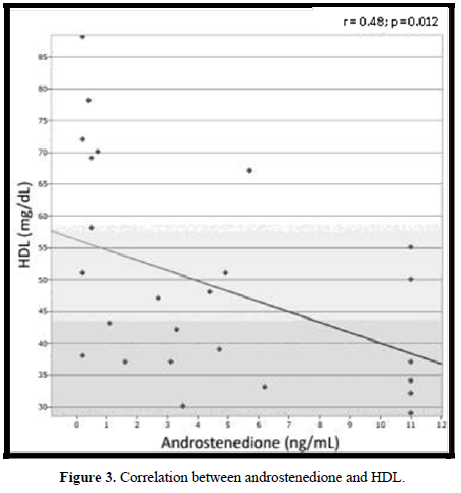
In 18 (72%) patients, the lipid profile was altered (Figure 2). No correlation was observed between the lipid parameters and the chronological age, equivalent dose of hydrocortisone, z- BMI, and adiponectin. Moreover, no difference was detected between the sexes or the type of glucocorticoids used for the treatment. The levels of LDL were higher in salt-wasting patients than in simple virilizing patients (93 mg/dL vs 64.4 mg/dL, respectively; p = 0.042), although the median was within the normal range. A significant negative correlation was observed between the levels of HDL and androstenedione (r = -0.48; p = 0.012), whereas a positive correlation was detected between the levels of insulin and triglycerides (r = 0.47; p = 0.014) (Figures 3 & 4).
Only 1 (4%) child presented hyperinsulinemia, whereas 2 (8%) presented borderline serum levels of insulin. The levels of fasting glucose were normal in all patients. There were no differences in the average levels of glycemia and median insulin in relation to sex, clinical manifestations of CAH, or type of glucocorticoids used for the treatment. A significant positive correlation was detected between the levels of blood glucose and fasting insulin (r = 0.41; p = 0.041) (Figure 3). The levels of blood glucose and serum insulin did not correlate with the levels of androstenedione and adiponectin, age, or equivalent dose of hydrocortisone.
The serum levels of adiponectin were higher in the group treated with hydrocortisone, followed by patients receiving prednisone/prednisolone, and was lower in the group treated with dexamethasone (19.71 µg/dL vs 12.34 µg/dL vs and 7.67 µg/dL, respectively; p = 0.047). No difference in the median levels of adiponectin was observed between the sexes and the clinical manifestations of CAH.





DISCUSSION
The concern to trace metabolic disorders in patients with CAH has been gaining attention in recent years [6-10,13]. During the past 60 years, the increased availability of glucocorticoids for use in the treatment of CAH has increased the long-term survival of patients. Therefore, more attention has been given to the possible health problems developed in adulthood that are related to the disease, and the possible appearance of signs and symptoms of these comorbidities already in childhood. This study showed a high prevalence of obesity, dyslipidemia, and hypertension in children and adolescents with CAH.
The prevalence of obesity in our study was 16%, which is consistent with the findings in the literature that varies between 16% and 35% [11,12,23]. Despite an increase in childhood obesity in Brazil, the prevalence of obesity in patients with CAH is even higher than that observed in Brazilian children between 5 and 19 years old, according to the “Family Budget Survey 2008-2009” carried out by the Brazilian Institute of Geography and Statistics [24]. Although several studies have correlated z-BMI with the equivalent dose of hydrocortisone [11,23] and the use of corticosteroids with longer half-life [23], in our study, no correlation was observed between z-BMI and the dose or type of glucocorticoids used for the treatment, presumably because 87% of our patients were using glucocorticoids with a half-life longer than that of hydrocortisone.
In our study, the frequency of dyslipidemia was higher than that observed by most authors [12,23,25,20]. Of our patients, 72% presented several changes in their lipid profile, and a mild negative correlation was observed between the levels of HDL and androstenedione (r = -0.48; p = 0.012). This may suggest that an inappropriate disease control could represent a risk factor for dyslipidemia. Although the dyslipidemia in CAH patients could be justified by the hyperinsulinemia caused by both hypercortisolism and hyperandrogenism [15,20], in our group, only 1 (4%) patient presented an increased level of insulin. No correlation was observed between the serum levels of insulin and those of HDL, LDL, and TC. A mild positive correlation was observed between the levels of insulin and those of triglycerides (r = 0.47; p = 0.014).
In our study, the prevalence of patients with increased BP was 32%. BP was ≥90th percentile in 24% of the patients and ≥95th percentile in 8%. This prevalence was higher when compared with that of Brazilian children, which varies from 0.8% to 8.2% [26,27]. When divided into systolic and diastolic pressures, 16% presented SBP above normality, according to age and sex (8% had increased SBP and 8% were borderline), whereas a borderline SBP was detected in 16% of the patients. A limitation to our study is that BP was measured during a medical appointment and not by monitoring the patients, which could increase the possibility of “white coat hypertension”. However, the frequency of hypertension and prehypertension was lower in our group than in the patients described by Subbarayan and collaborators [12], whose frequencies of increased SBP and DBP were of 20.9% and 11%, respectively, obtained by monitoring the BP for 6 h. The levels of SBP or DBP did not correlate with the equivalent dose of hydrocortisone and plasma renin activity. Although obesity is a known risk factor for systemic hypertension [27-28], in our group, a higher frequency of altered BP was not detected in overweight patients and no correlation was observed between SBP and DBP and z-BMI. Our results are similar to those published by Subbarayan and collaborators [12] and by Finkielstain and collaborators [23].
Our study was the first to evaluate the WC in a pediatric population with CAH. WC is considered an independent predictor of cardiovascular risk in adults and children. It is an important indicator of insulin resistance, dyslipidemia, and hypertension [19,28,29]. In our study, 48% of the patients presented increased WC, which positively correlated with SBP. Moreover, a significant negative correlation was observed with the serum levels of adiponectin, considered a protective factor for cardiovascular disease [30-32]. Studies with higher numbers of patients would be required to assess whether an increased WC is an independent risk factor for cardiovascular disease in children with CAH.
Only 1 study evaluated the serum levels of adiponectin in children with CAH [16]. The authors found a negative correlation with z-BMI, age, and pubertal stage, whereas no difference was observed in the levels of adiponectin between the different types of glucocorticoids used for the treatment. In our study, no correlation was observed between the levels of adiponectin and these variables. However, a difference between the median levels of adiponectin and the type of glucocorticoids used for the treatment was detected, being higher in the group receiving hydrocortisone, followed by the group treated with prednisone/prednisolone, and lower in patients receiving dexamethasone. Several studies show that glucocorticoids decrease the levels of adiponectin. Degawa-Yamauchi and collaborators [32] reported a diminished release of adiponectin in adipocytes after infusion with dexamethasone. Fallo and collaborators [33] already observed that lower levels of adiponectin were detected in patients with Cushing’s syndrome. Dexamethasone is a potent glucocorticoid with a longer half-life than hydrocortisone, which may account for the lower levels of adiponectin in patients receiving dexamethasone and for the higher levels of adiponectin in patients treated with hydrocortisone. However, further studies would be required to assess the real interference of the type of glucocorticoids used for the treatment in the metabolism of adiponectin in patients with CAH.
In this study, the high prevalence of obesity, overweight, hypertension, and dyslipidemia that were observed would justify the early assessment of risk factors for cardiovascular disease in pediatric patients with CAH. WC positively correlated with systolic BP and negatively with adiponectin. The serum levels of HDL already negatively correlated with androstenedione.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests that could be perceived as prejudicing the impartiality of the study.
FUNDING
This research did not receive any specific grant from any funding.
- Marumudi E, Khadgawat R, Surana V, Shabir I, Joseph A, et al. (2013)Diagnosis and management of classical congenital adrenal hyperplasia. Steroids 78: 741-
- Wedell A (1998) Molecular genetics of congenital adrenal hyperplasia (21-hydroxylase deficiency): Implications for diagnosis, prognosis and treatment. Acta Paediatric 87: 159-164.
- Joint Lwpes/Espe Cah Working Group (2002) Consensus statement on 21-hydroxylase deficiency from The Lawson Wilkins Pediatric Endocrine Society and The European Society for Pediatric Endocrinology. J Clin Endocrinol Metab 87: 4048-
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, et al. (2010) Endocrine Society, “Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline”. J Clin Endocrinol Metab 95: 4133-
- Kim MS, Ryabets-Lienhard A, Geffner ME (2012)Management of congenital adrenal hyperplasia in childhood. Curr Opin Endocrinol Diabetes Obes 19: 483-488.
- Reisch N, Arlt W, Krone N (2011) Health Problems in Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency.Horm Res Pediatr 76: 73-
- Speiser PW, White PC (2003)Congenital adrenal hyperplasia.N Engl J Med 349: 776-
- Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, et al. (2007) Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency.Horm Res 67: 268-276.
- Nebesio TD, Eugster EA (2006)Observation of hypertension in children with 21- hydroxylase deficiency: a preliminary report.Endocrine 30: 279-
- Hoepgffner W, Hermann A, Willgerodt H, Keller E (2006) Blood pressure in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency.J Pediatr Endocrinol Metab 19: 705-
- Völkl TM, Simm D, Beier C, Dorr HG (2006) Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency.Pediatrics 117: 98-105.
- Subbarayan A, Dattani MT, Peters CJ, Hindmarsh PC (2014) Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol 80: 471-477.
- Charmandari E, Chrousos GP (2006) Metabolic syndrome manifestations in classic congenital adrenal hyperplasia. Do they predispose to atherosclerotic cardiovascular disease and secondary polycystic ovary syndrome?Ann N Y Acad Sci 1083: 37-
- Mooij CF, Kroese JM, Grinten HRC, Tack CJ, Hermus RMM (2010) Unfavorable trends in cardiovascular and metabolic risk in pediatric and adult patients with congenital adrenal hyperplasia?Clin Endocrinol 73: 137-
- Strohmayer EA, Krakoff LR (2011) Glucocorticoids and cardiovascular risk factors. Endocrinol Metab Clin North Am 40: 409-
- Volkl MKT, Simm D, Korner A, Kiess W, Kratzsch J, et al. (2009) Adiponectin levels are high in children with classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Acta Pædiatrica, 98: 885-
- World Health Organization. The WHO Child Growth Standards. Available online at: http://www.who.in
- National Health and Nutrition Examination Survey (2004) Anthropometry Procedures Available online at: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf
- Fernandez JR, Redden DT, Pietrobelli A, Allison DB (2004) Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 145: 439-4
- Brazilian Society of Cariology (2005) Guidelines for the prevention of atherosclerosis in childhood and adolescence of the Brazilian Society of Cardiology. Braz Arch Cardiol 85: 1-
- Stewart MP, Krone PN (2011) The adrenal cortex. In: S. Melmed, K. S.Polonsky, P. R. Larsen, H. M. Kromemberg (Org). Williams Textbook of Endocrinology. 12th edn, Philadelphia: Elsevier, pp: 479-534.
- Madeira R, Carvalho CNM, Gazolla FM, Matos AH, Borges MA, et al. (2008) Cut-off point for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index established from Receiver Operating Characteristic (ROC) curve in the detection of metabolic syndrome in overweight pre-pubertal children.Arq Bras Endocrinol Metabol 52: 1466-
- Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Ryzin CV, et al. (2012) Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 97: 4429-
- Oliviera LMB, Faria JAD, Silva DN, Lago R, Toralles MBP (2013) Elevated levels of leptin and LDL-cholesterol in patients with well controlled congenital adrenal hyperplasia.Arq Bras Endocrinol Metabol, 57: 354-
- Zimmermann A, Grigorescu-Sido P, AlKhzouz C, Patberg K, Bucerzan S, et al. (2010) Alteration in lipid and carbohydrate metabolism in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr 74: 41-
- Rahmouni K, Correia MLG, Haynes WG, Mark AL (2005) Obesity-associated hypertension: New insights into mechanisms. Hypertension 45: 9-
- Damiani D, Kuba VM, Cominato L, Damiani D, Dichtchekenian V, et al. (2011) Metabolic Syndrome in children and adolescents: Doubts about terminology, but not about cardiometabolic risks. Arq Bras Endocrinol Metabol 55: 576-
- Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tato L (2001) Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res 9: 179-
- Prins B (2002) Adipose tissue as an endocrine organ. Best Pract Res Clin Endocrinol Metab 16: 639-
- Costa JV, Duarte JS (2006) Adipose tissue and adipokines. Portuguese Medical Acta 19: 251-
- Madeira AR, Carvalho CNM, Gazolla FM, Pinto LW, Borges MA, et al. (2009)The impact of obesity on metabolic syndrome components and adipokine levels in prepubertal children. J Pediatr 85: 261-
- Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, et al. (2005) Regulation of adiponectin expression in human adipocytes: Effects of adiposity, glucocorticoids, and tumor necrosis factor alpha.Obes Res 13: 662-
- Fallo F, Scarda A, Sonino N, Paoletta A, Boscaro M, et al. (2004) Effect of glucocorticoids on adiponectin: A study in healthy subjects and in Cushing's syndrome. Euro J Endocrinol 150: 339-
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Pathology and Toxicology Research
- Journal of Rheumatology Research (ISSN:2641-6999)
- Chemotherapy Research Journal (ISSN:2642-0236)