2898
Views & Citations1898
Likes & Shares
It is well known that articular cartilage
(AC) lacks the ability to repair itself once damage, thereby making it
therapeutic treatment challenging. A number of efforts are been made to induce
adult stem cells with growth factors or bioactive molecules for their
characterisation and mechanisms involved in their chondrogenic differentiation.
This study investigated the effect of Pleurostylia
capensis (P. capensis) bark and
root extracts on chondrogenic differentiation of porcine adipose-derived
mesenchymal stem cells (pADMSCs). The effect of P. capensis bark and root extracts at 5, 15, 30 and 50 µg/mL and
TGF-β3 (10 ng/mL) as positive control on cellular growth viability and
behaviour of pADMSCs was investigated using MTT and xCELLigence assays. The
biosynthesis of glycosaminoglycan (GAG) and the expression of chondrogenic
markers SOX 9, aggrecan (AGG), proteoglycan (Proteo), collagen type II (Col
II) and X (Col X) of pADMSCs in
pellet culture was investigated in vitro.
The results showed that P. capensis
bark extracts at 5 and 50 µg/mL stimulated the proliferation of pADMSCs from 24
to 48 h of incubation with cell viability of about 100%, and the root extracts
showed cell viability of about 90% with all treatments at 48 h. The amount of
GAG synthesised was high with bark extracts at 5 and 15 µg/mL and with root
extracts at 15 and 30 µg/mL over both control and TGF-β3 at 21 days. Bark
extracts at 30 µg/mL induced the highest expression of SOX 9, Proteo, Col II and Col X significant at p˂0.01 at 14 days. Whereas, root extracts at
15 µg/mL induced the highest expression of SOX
9 and AGG at 14 days. All the
cells treated with P. capensis bark
and root extracts displayed a strong positive stain for Safranin-O and strongly
observed Toluidine blue at day 14. Immunohistostaining revealed little positive
staining at matrix for COL-10 from both groups of treatments. Nevertheless, P. canpensis bark at 30 µg/mL and root
extracts at 15 µg/mL is likely to be a future treatment strategy for
chondrogenic differentiation of stem cells, and supports the use of this plants
extracts as used in indigenous knowledge.
Keywords: Pleurostylia capensis,
Glycosaminoglycan, Extracellular matrix, Adipose-derived mesenchymal stem cell
INTRODUCTION
Articular Cartilage
(AC) injury and deterioration are predominant in athletes, obese and ageing
populations and results from chronic joint stress or acute traumatic injuries
[1]. Due to the limited healing capacity of AC caused by vascularity and
self-repair for cartilage, it is hard to repair without external treatments
after injury. Injury to cartilage is a major risk factor for early development
osteoarthritis (OA) [2-7]. OA is a joint disease that is commonly defined as
the erosion of joint cartilage but in reality, affects multiple tissues of the
joint including the ligaments, bone, synovium and meniscus (if the joint
involved is the knee) as more recently redefined by the Osteoarthritis Research
Society International (OARSI) [8]. Repetitive loading of AC activities can lead
to progressive articular cartilage degradation with an accumulation of
catabolic enzymes, cytokines, fragmentation of collagen and aggrecan as well as
a progressive breakdown of the articular surface [9].
Many surgical
(microfracture, abrasion arthroplasty, osteochondral autologous, autologous
chondrocyte implantation (ACI), matrix-induced autologous chondrocyte
implantation (MACI) and subchondral drilling), cell transplantation (stem cell
or chondrocyte implants) and medical treatments have been described with the
common goal of improving joint function and halting disease progression
[10-12]. These techniques were designed to repair cartilage with the production
of hyaline cartilage formation. Despite
increased research
on the treatment
Currently,
the research focus has shifted to investigating the use of adult mesenchymal
stem cells (MSCs) in tissue engineering and regenerative medicine for cartilage
repair, because of their self-renewal capacity and their ability to
differentiate along multiple lineages including chondrocytes [13,14]. MSCs are
used to repair and replace tissues or organs that are damaged for cell-based
therapies [15-18]. In general, stem cell behaviors, such as attachment,
proliferation, and differentiation into specific lineages is dependent on a
multitude of physical, chemical and environmental factors, including substrate
topography, extracellular matrix’s (ECM), stem cell growth factor/chemical
inducer interactions and stem cell-substrate interactions [19].
This study, aimed to investigate the
influence of Pleurostylia capensis
water extracts (bark and root) as plant-based morphogenetic factors on porcine
adipose-derived mesenchymal stem cells (pADMSCs). The cellular behavior,
proliferation and differentiation of pADMSCs into chondrogenic lineages was
studied using the amount of proteoglycan secreted, the deposition of GAG and
expression of chondrogenic markers such as SOX
9, aggrecan, proteoglycan, collagen type II and X. It was hypothesised that
the treatment of pADMSCs with P. capensis
crude extracts would stimulate the proliferation rate and chondrogenic
differentiation of pADMSCs.
MATERIALS AND METHODS
Plant collection and selection
Pleurostylia capensis bark
and root materials were selected based on its ethno pharmacological use in the
management of osteoarthritis, mode of preparation and administration by
traditional healers, and the absence of published literature describing their
effects on pADMSCs differentiation into chondrocytes by gene profiling.
Medicinal plants were collected at Limpopo province in the Venda region of
South Africa during March 2016 (summer season). The plant materials collected
were barks and roots. The materials were sent to the botanist at the University
of Venda for identification and voucher specimen number was given (MPT0060).
Preparation of extracts
Collected bark and root materials were washed
with water to remove soil and then placed in a shade to dry at room temperature
for about two weeks. The dried materials were ground to powder form (mechanical
blender, ATO MSE mix) and kept in airtight polyethylene bags until needed for
extraction purpose. Tiwari et al. [20], describe the extraction method used
with minor modification. About 50 g of powdered plant material was dissolved in
500 mL of distilled water. The mixture was shaken vigorously for 24 h at room
temperature. The mixture was filtered after which it was frozen for overnight.
The frozen materials were dried under freeze dryer to get the crude extracts.
The crude extracts were used for various biological assays.
Isolation and culture of stem cells
The porcine adipose-derived mesenchymal stem
cells (pADMSCs) was isolated from the stifle (knee) joint of 3 month old
porcine that was obtained within 6 h of slaughter from a local abattoir.
Isolation of MSCs from adipose tissue was performed as previously described by
Khan et al. [21] with minor modification. The fatty tissue was washed twice
with PBS. Subsequently, the tissues were minced and digested with 0.15% m/v
type II collagenase (Invitrogen, Carlsbad, CA) at 37°C for 45 min. The
suspension was centrifuged at 1500 rpm for 10 min. The supernatant was removed,
the pellet was washed twice with alpha-minimal essential medium (α-MEM)
containing 10% FBS (v/v) and suspended in 1 ml of α-MEM/10%FBS v/v/5 ng/ml
FGF-2. The suspension was filtered through 50 μm nylon-mesh strainer to remove
fibrous debris. Cells were grown to confluence (80%). The medium was changed
for the first time after three days. The cell monolayer was washed with PBS and
was detached with 0.05% v/v trypsin-EDTA. Cells were counted and assigned to
different assays at passage zero (P0).
Cell culture for proliferation
Cellular
proliferation and behavior were performed by plating 100 µL 2 × 104
cells/mL in 96 well plates and a special plate E-plate 16 overnight at 37°C
with 5% CO2. After incubation, the media was aspirated and 100 µL P. capensis bark and root extracts at 5,
15, 30, 50 and 100 µg/mL was added to each well with three replicates. The
dosage was determined based on our previous study [22]. TGF-β3 was used as a
positive control at 10 ng/mL as used in previous study [23]. The plates were
incubated for 24, 48 and 72 h and at the end of each incubation time an MTT
assay and cellular behavior test using the xCELLigence system was performed and
an optimal concentration was selected for further analysis.
Cell culture for chondrogenic differentiation
pADMSCs were
cultured in a pellet model and investigated for chondrogenic differentiation.
For this, 5 × 105 of pADMSCs were centrifuged at 1500 rpm for 5 min
to make cell pellets in 1.5 mL sterile conical micro tubes with removable
screw-type lids. The pellets were then cultured in 0.5 mL 1% FBS/complete α-MEM
and P. capensis (bark and root
extracts) at 5, 15 and 30 µg/mL and 10 mg/mL TGF-β3 at 37°C in a humidified, 5%
CO2 tissue culture incubator. The medium was changed every three
days, and pellets were harvested on day 14 and 21. At the end of each culture
stage, the cell pellets were assessed biochemically for their glycosaminoglycan
(GAG) matrix and DNA contents. This was done histologically for the GAG matrix
using immunohistological staining for cartilage-specific matrix proteins and by
real-time quantitative reverse transcription polymerase chain reaction
(qRT-PCR) for the gene expression analysis.
Viability analysis for pADMSCs
The cellular
viability was assessed indirectly, by quantifying the cellular conversion of a
tetrazolium salt (MTT) into a formazan product. The behaviour of the cell was
investigated in time-dependent cells response impedance using the xCELLigence
System (RTCA DP Instrument, ACEA Biosciences, Inc.). The CI value at each time
point is defined as the (Rt-Rb)/15 where Rt is the cell-electrode impedance of
the well with the cells at different time points, and Rb is the background
impedance of the well with the media alone. The normalised cell index was
calculated by dividing the cell index value at a particular time point by the
cell index value at the time of interest.
Biochemical analysis
After 14 and 21
days of pellet culture, pellets were rinsed with 500 μL DPBS to remove any
residual medium and then digested in 250 μL proteinase K (1 mg/mL in Tris EDTA
buffer) overnight at 56°C. The GAG content was measured spectrophotometrically,
using one, 9-dimethyl methylene blue (DMMB) (Sigma-Aldrich); metachromatic cationic
dye, which binds to anionic GAG molecules. The degree of metachromaticity is
directly proportional to the amount of GAG present in the reaction mixture. GAG
was calculated as µg/mL of chondroitin-4-sulfate (CS) equivalents. The DNA
content was determined using the CyQuant cell proliferation assay Kit
(Invitrogen, ON, Canada) with the supplied bacteriophage λ DNA as a standard.
Gene expression analysis of pADMSCs
Total RNA was
isolated from two-time points (14 and 21 days) using NucleoSpin® RNA
kit according to the manufacturer’s instructions (MACHEREY-NAGEL, Düren,
Germany). RNA (100 ng) was reverse transcribed to cDNA by iScript Reverse
transcriptase (Bio-Rad Laboratories, Inc.). Reverse-transcription quantitative
polymerase chain reaction was performed in a DNA Engine Opticon I Continuous
Fluorescence Detection System (Bio-Rad, CA, USA) using hot start iTaq Universal
SYBR Green Super mix (Bio-Rad Laboratories, Inc.). Primers sequences were
obtained from Inqaba biotech (Table 1).
Expression levels of mRNA relative to the control (untreated) culture were
calculated using the threshold cycle (∆CT).
Histology of pad MScs chondrogenesis
After 14 and 21
days of pad MScs being treated with plants extracts in the micro mass pellet,
the media was removed from the cells pellet, fixed overnight in 10% formalin,
dehydrated and embedded in paraffin wax. Section of 5 μm thickness was cut and
stained with 0.01% (w/v) Safranin-O and 1% (w/v) Toluidine blue to reveal the
GAG matrix deposition [24].
Immunohistological staining of pad MScs chondrogenesis
The sections
were deparaffinised, rehydrated, then pre-treated with proteinase K, endogenous
peroxidase activity block with 3% hydrogen peroxide in PBS and incubated with
primary antibody anti-collagen type X (COL-10) ab49945 (Abcam) and incubated
for 1 h at room temperature. A One-Step Polymer-HRP reagent (Biogenex Super
Sensitive One-Step Polymer-HRP Detection System) was applied to the section and
it was incubated for 15 min. A 200 µL volume of DAB substrate solution
(Biogenex Super Sensitive One-Step Polymer-HRP Detection System) was added to
each section. The slides were counterstain with Mayer’s hematoxylin and mounted
with paramount.
STATISTICAL ANALYSIS
The experiments
were performed using three biological replicates (N=9), with a minimum of three
technical replicates for each experimental time point. The statistical analysis
was performed using Graph-Pad Prism 5; a one-way ANOVA followed by Bonferroni’s/Dennett
multiple comparison tests. For this ***p<0.001, **p<0.01, *p<0.05 was
considered significant.
RESULTS
Cellular proliferation and behavior
Cell viability and cell cytotoxicity were
used to evaluate whether P. capensis
extracts affected the cellular growth of pad MScs cultured in vitro and to select optimal concentrations that supported pad
MScs growth better at about 80%. Basic
toxicology, the LD50 value is defined as the statistically derived dose that,
when chemical or substances are administered in a cell of test, is expected to
cause death in 50% of the treated cells in a given period [25]. Isolated pad MScs showed typical
fibroblastic morphology on days 1, 4 and 7 after treatment with P. capensis extracts inoculation and
grew in whorl-like and myoblast formation. The viability of pad MScs cells was
assessed using MTT assays based on the cellular conversion of tetrazolium salts
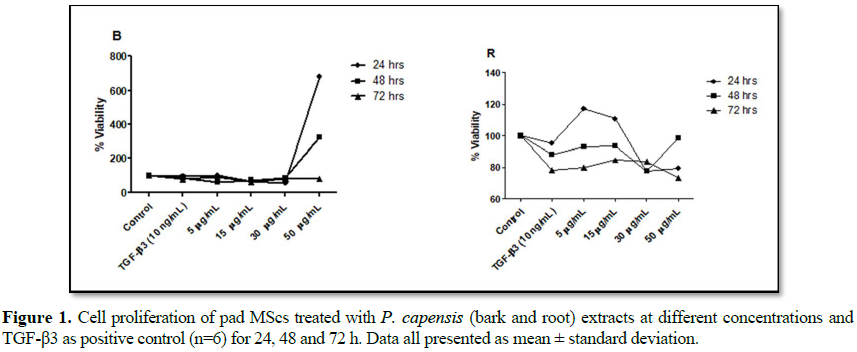
into Formosan products. The results showed cell viability above 100% at 48 h
with bark extracts at 5 and 50 µg/mL, while 15 and 30 µg/mL showed viability at
about 80%. At 72 h, the bark extracts showed cell viability of 67% with all
treatment (Figure 1). TGF-β3 at 10
ng/mL showed cellular viability at about 90% at 48 h, the proliferation rate
was declined by 12% at 72 h. The root extracts at 48 h showed cellular
viability of about 90% and at 72 h, the viability was decreased by 18% with all
the treatments (Figure 1). These
results suggest that the plant materials were not toxic to the cells as all
treatments possessed inhibition concentration (IC) of about 33%. Cellular
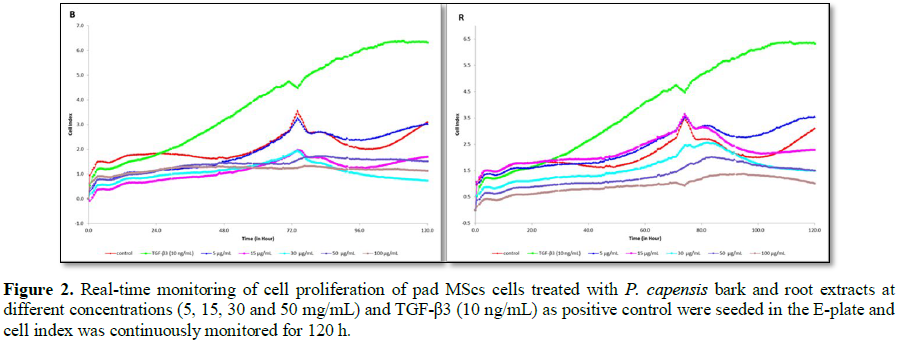
behavior of pad MScs after treatment with different concentrations of P. capensis bark and root extracts was
monitored with the xCELLigence system measuring CI at a given time (Figure 2). The CI was proportional to
the number of adherent cells remaining on the E-plate at 24 h. As shown in Figure 2, TGF-β3 showed an increase in
CI until 120 h compared to the untreated cells. The bark extracts at 5, 15 and
30 µg/mL showed increased in CI until 72 h, these results correspond with MTT
results shown in Figure 1. Root extracts
showed a trend increased in CI until 120 h with 5, 15, 30 and 50 µg/mL. This
result indicates that the cells were proliferating, as the graph does not show
CI below zero as indication of toxicity (Figure
2). There was a reduction in CI at 50 and 100µg/mL of bark extracts and 100
µg/mL of root extracts.
Biochemical analysis
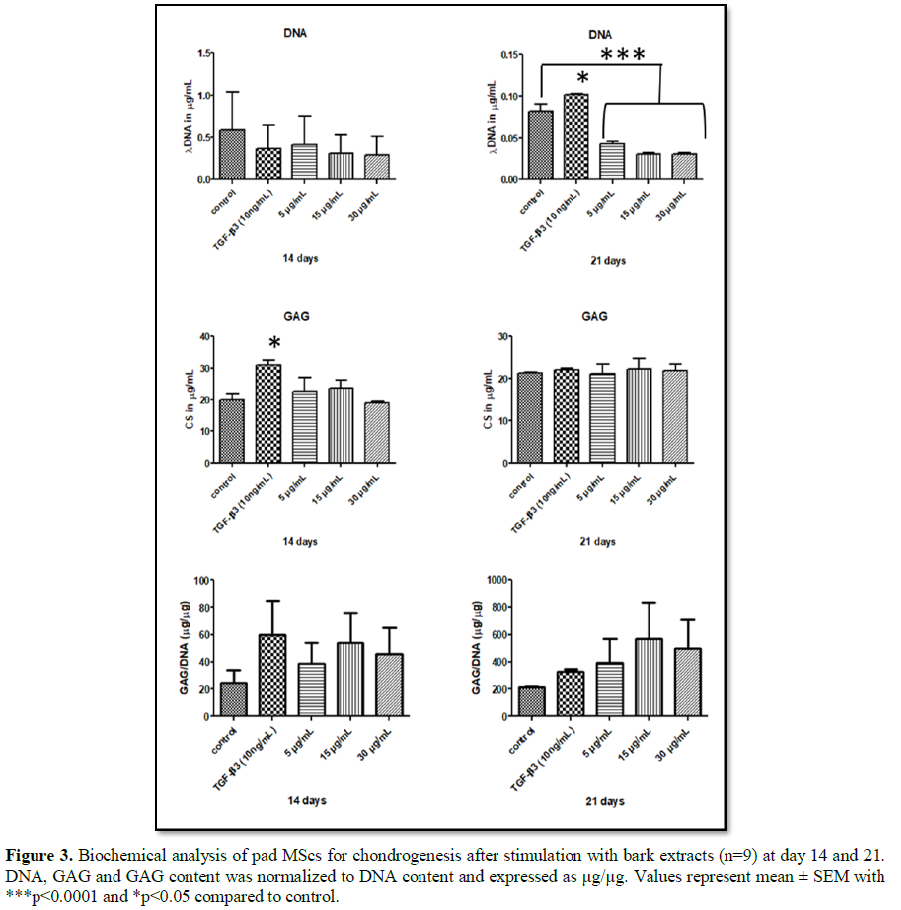
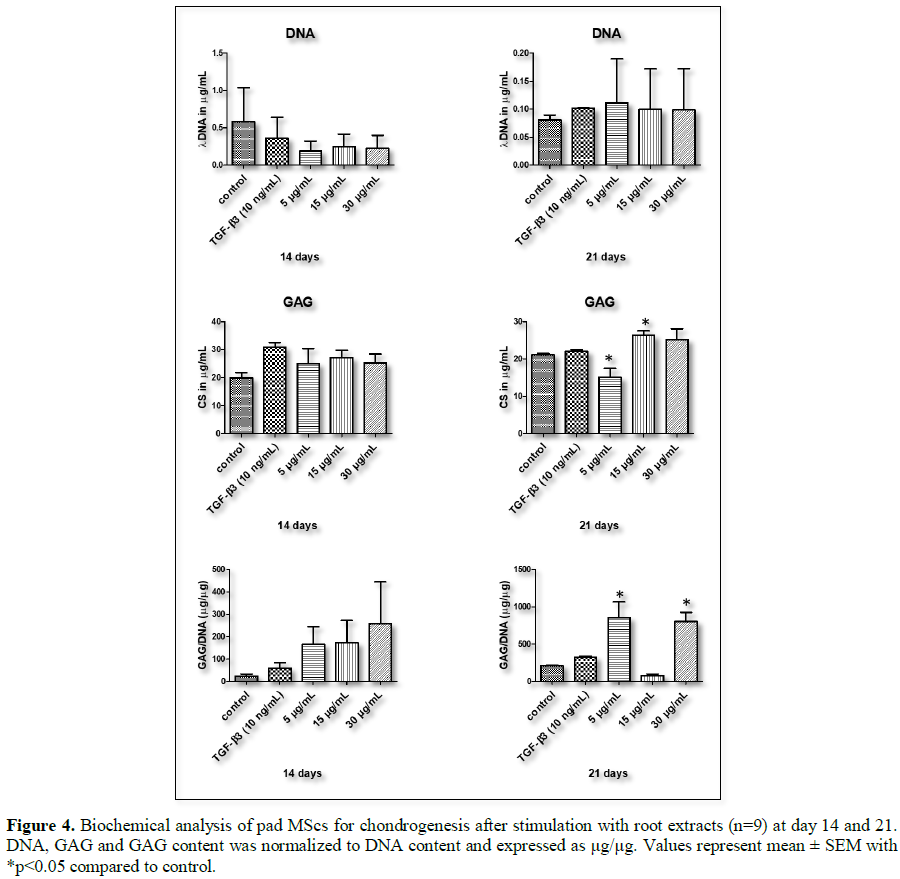
Biochemical
analysis was performed to assess the synthesis of glycosaminoglycan chondroitin
sulphate (CS), DNA content and GAG/DNA ratio at 14 and 21 days of pad MScs
chondrogenic differentiation. P. capensis
bark at 5 and 15 µg/mL treatment showed a higher synthesis of GAG matrix when
compared to control (Figure 3). We
have observed a decrease in GAG matrix synthesis by P. capensis root at 5 µg/mL at day 21 of treatment. However, root
extracts at 15 and 30 µg/mL showed a higher synthesis of GAG matrix when
compared to both control and TGF-β3, which is a positive control as shown in Figure 4. DNA content was lower than
control in all the treatment groups at 14 and 21 days. GAG/DNA (µg/ng) ratio
shows a slight increase of above 0.5 at 21 days with all treatments as shown in
Figure 3. Pleurostylia capensis root extract at 5 µg/mL showed a decrease in
DNA content from day 14 to 21 (Figure 4).
There was a slight decrease in the synthesis of GAG by P. capensis root extract from 14 to 21 days.
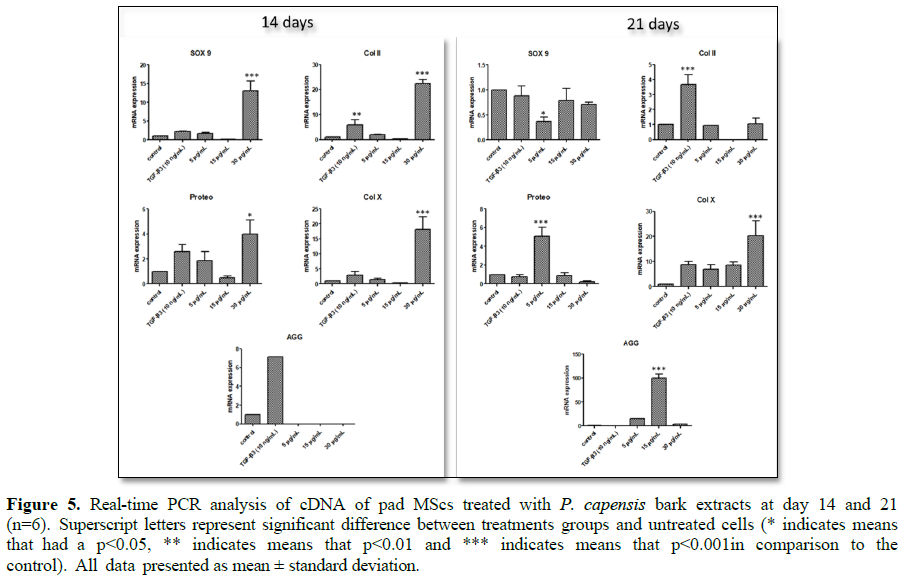
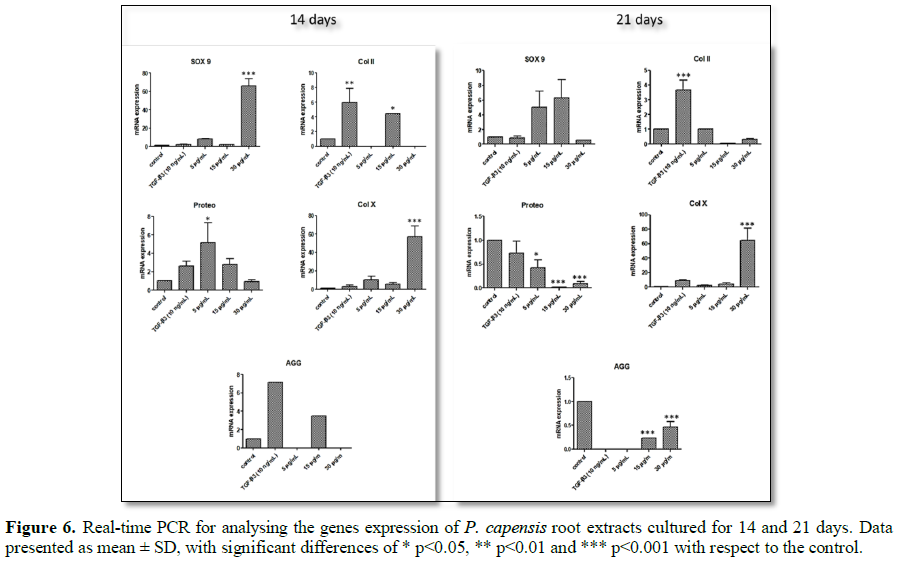
Gene expression analysis
To characterise
the ECM generated in pellets, the expression of chondrogenic markers was
assessed by qRT-PCR on day 14 and 21. The expression of SOX 9, Proteo, Col II and Col X was significantly up-regulated in 30 µg/mL bark extract
compared to the negative control and TGF-β3 at day 14 (Figure 5, p<0.01), while the expression of these genes at day 21
was significantly down regulated. However, mRNA expression of SOX 9 and Col X was up regulated by a five-fold increase in 30 µg/mL root
extracts at day 14 (Figure 6, p<0.001).
Root extracts at 15 µg/mL showed an increase in the expression of AGG, Preteo
and Col II at day 14 of the pellet
culture compared to control (Figure 6).
The root extracts at 30 µg/mL significantly up-regulated Col X and SOX 9 at day 14. TGF-β3 showed up-regulation of AGG at
day 14 and Col II at both day 14 and
21. AGG was not expressed by
bark at 5, 15 and 30 µg/mL and roots extracts at 5 and 30 µg/mL on day 14. At 21 days, TGF-β3 and root extracts at 5 µg/mL showed no
expression of AGG marker. The relative gene expression
of Col X remained stable with no
significant change with bark and root at 30 µg/mL until 21 days. There was an
up-regulation of proteoglycan by day 21 with a one-fold increase for 5 µg/mL
bark and root extract at 14 days.
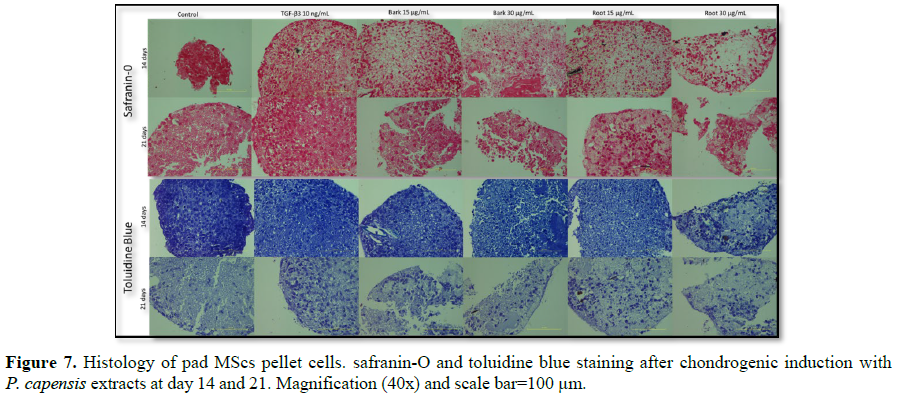
Histology and immunohistochemistry analysis
After two and three
weeks of pellet culture in chondrogenic medium supplemented with P. capensis bark and root extracts at 15
and 30 µg/mL, pellets were embedded, cut and stained with Safranin-O and
Toluidine blue for GAG deposition (pink/red and blue staining). Positive
staining was identified in all treatment groups with higher intensity on day 14
(Figure 7). Toluidine blue stain
showed strong staining in 15, 30 bark extract and 15 µg/mL root extract. Weaker
blue staining was observed in cells cultured in 30 µg/mL root extract by 14
days, compared to the control groups. At 15 µg/mL bark extract, Safranin-O
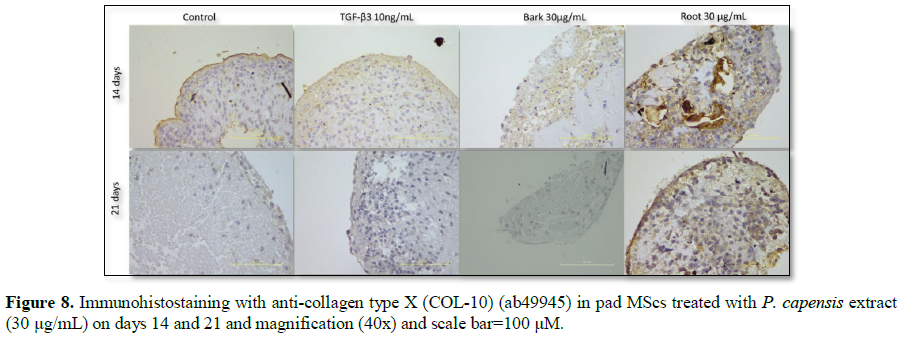
positive proteoglycan was more intense. Staining for Collagen Type X as a
marker for chondrocytes hypertrophy is illustrated in Figure 8. Pellet from both groups of cells showed little positive
staining at matrix of COL-10 on day 14. As shown in Figure 6, bark extracts at 30 µg/mL showed no staining of COL-10
and pellet from root extracts at 30 µg/mL showed per cellular staining at day
21, these were caused by pellet size that were very small.
DISCUSSION
In this study, the effect of plant-based
morphogenetic protein stimulators of P.
capensis extracts (bark and root) on chondrogenic differentiation of pad
MScs after treatment for 21 days was investigated. This work forms part of a
broad research project aimed at finding alternative plant-based induction of
stem cells for AC repair. AC is a tissue that is avascular, a neural and a lymphatic,
with limited ability to regenerate once damaged, because of a lack of blood
supply to facilitate cells and factors that promote healing [26-29]. It was found that P. capensis root extracts were good
inducers of stem cell proliferation, with about 90% of cell viability (Figure 1) at 48 h of treatments.
Similar observation was noted with TGF-β3 at 10 ng/mL used as positive control.
Whereas, bark extract at 5 and 50 µg/ml showed cell viability of about 100% and
15 and 30 µg/ml with about 80% at 48 h. These results suggest the possibility
of using medicinal plants as a source of inducers for stem cell proliferation.
The cellular behavior of pad MScs induced with P. capensis bark and root extracts was monitored using the
xCELLigence system based on the number of cell adherent to the embedded
electrode E-plate as CI value. P.
capensis bark and root extracts were found to be nontoxic to the cells,
with a Cell index value above one. The results observed with the MTT assay
corresponded with those of the xCELLigence system, with root extracts being the
most effective compared to bark extracts. The results indicated the biological
status of the cells, including their cellular behavior in response to
treatments.
Cellular nucleic acid content is a reasonable
indicator of cell number since the levels of DNA and RNA in cells are highly
regulated. Although the DNA levels of individual cells change over time, the
net nucleic acid content per cell in a non-synchronous culture typically
remains relatively constant [30,31]. The metabolic activity may,
however, be changed by different conditions or chemical treatments which can
cause considerable variation in the results from these assays [32]. The GAG results showed root
extracts at 15 and 30 µg/mL resulted in a significant 76% increase in GAG
production over control and TGF-β3 at 10 ng/mL (Figures 3 and 4). However, bark extracts showed 30% GAG synthesis
increased at 5, 15 and 30 µg/mL compared to control cells. TGF-β3 results were
similar to the one from Hoben et al. [33] with 60% increase in GAG synthesis
compared to control. The results showed change in the physiological levels of
the cartilage GAG which is thought to be important to chondrogenesis and to
normal skeleton formation [34].
The expression of cartilage-specific genes,
including SOX 9, Col II, Col X, AGG and
Proteo was analysed for chondrogenic differentiation in pad MScs treated with
TGF-β3 and P. capensis bark and root
extracts. The results showed high expression of SOX 9, Col II, Col X and Proteo by bark 30 at 14 days of treatment over
control and TGF-β3. However, root extracts showed high expression of SOX 9,
Proteo, Col X at 14 days. At day 21, P. capensis bark and root extracts
showed decrease in the expression of Col II marker compared to TGF-β3, while
bark at 30 µg/mL expressed Col II over root extracts at all concentrations.
Hyaline cartilage contains at least three tissue-specific collagens, types II,
IX and XI, with collagen types II representing 95% of ECM and forming fibril
interconnected with proteoglycan aggregate [35-38]. The results showed SOX 9 a transcription factor expressed
more by root extracts at 5 and 15 µg/mL compared to both control and TGF-β3 at
21 days (Figure 6). SOX 9 is expressed in the developed cartilage
from the skeletogenic progenitor stage and remains expressed in the
chondrocytes until hypertrophy throughout adult hood in AC [39-41]. The deposition of GAG matrix was
more intense at day 14 when staining with Safranin-O AND toluidine blue. The
intensity of the stain change at day 21 with all the treatments. The
localization of COL-10 was done to determine if the differentiation undergoes
hypertrophic. It was interesting to note that Col X, the key factor associated with hypertrophy expression,
remained the same at 30 µg/mL on days 14 and 21 (Figures 5 and 6). Correspondingly, immunohistostaining of 30 µg/mL
root extract revealed strong positive staining of COL-10 (Figures 7 and 8).
CONCLUSION
To the best of
the researchers understanding, this study introduced the proliferative and
chondrogenic effect of P. capensis
bark and root crude extracts in adipose tissue-derived MScs. In conclusion,
root extracts were more potent than bark extracts as a potential source of
bioactivity for chondrogenesis of pad MScs. The root extracts showed virtuous
cellular behavior, proliferation and differentiation of pad MScs into
chondrogenic lineages. The root extracts demonstrated increased in GAG
production with minor different from bark extracts. Furthermore, chondrogenic
differentiation of pad MScs with P.
capensis root extracts at 15 and 30 µg/mL upregulated AGG, SOX 9 and Col X the markers for
chondrogenesis. P. capensis root at
30 µg/mL showed localization of hypertrophic marker COL-1O. These findings
suggest that root at 30 µg/ml has been probable mineralization zones of hyaline
cartilage with formation of bone like structure. P. capensis bark extract at 30 µg/mL showed expression of Col II, Proteo and SOX 9 at an early stage of
chondrogenic differentiation of pad MScs. This study extends the knowledge of
how pad MScs respond to P. capensis
bark, root crude extracts during chondrogenic differentiation and support the
use of this plant in indigenous settings for the treatment of diseases. Further
studies need to be carried out to extend on the understanding of mechanism in
which this plant use and which compounds are responsible for bone and
chondrogenic differentiation.
ACKNOWLEDGMENT
The authors
wish to acknowledge the financial support of the National Research Foundation
PhD Rating Track of South Africa, Mrs. Mpilu for sampling and use of materials
support and the Department of Biomedical Sciences, the Tshwane University of
Technology for financial and technical support.
CONFLICT OF INTEREST
The authors
declare no conflict of interest.
1. Luria
A, Chu CR (2014) Articular cartilage changes in maturing athletes: New targets
for joint rejuvenation. Sports Health 6: 18-30.
2. Berenbaum
F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not
osteoarthrosis!). Osteoarthritis and Cartilage 21: 16-21.
3. Ferguson
M, Collins R (2010) Knee injuries in football: Knee injuries are particularly
common in football. Continuing Medical Education 28: 202-206.
4. Kon
E, Filardo G, Brittberg M, Busacca M, Condello V, et al. (2018) A multilayer
biomaterial for osteochondral regeneration shows superiority vs. microfractures
for the treatment of osteochondral lesions in a multicentre randomized trial at
2 years. Knee Surg Sports Traumatol Arthrosc 26: 2704-2715.
5. Valdes
AM (2018) Chapter 24 - Osteoarthritis: Genetic Studies of Monogenic and Complex
Forms A2. In: Genetics of Bone Biology and Skeletal Disease (2nd Edn).
Edited by Whyte MP, Eisman JA, Igarashi T. Academic Press, pp: 421-438.
6. Ying
J, Wang P, Zhang S, Xu T, Zhang L, et al. (2018) Transforming growth
factor-beta1 promotes articular cartilage repair through canonical Smad and
Hippo pathways in bone mesenchymal stem cells. Life Sci 192: 84-90.
7. Monteiro
SO, Bettencourt EV, Lepage OM (2015) Biologic strategies for intra-articular
treatment and cartilage repair. J Equine Vet Sci 35: 175-190.
8. Blackburn
S, Rhodes C, Higginbottom A, Dziedzic K (2016) The OARSI standardised
definition of osteoarthritis: A lay version. Osteoarthritis and Cartilage 24:
192.
9. Mithoefer
K, Peterson L, Zenobi-Wong M, Mandelbaum BR (2015) Cartilage issues in football
- Today's problems and tomorrow's solutions. Br J Sports Med 49: 590-596.
10. Devitt
BM, Bell SW, Webster KE, Feller JA, Whitehead TS (2017) Surgical treatments of
cartilage defects of the knee: Systematic review of randomised controlled
trials. Knee 24: 508-517.
11. Erggelet
C, Vavken P (2016) Microfracture for the treatment of cartilage defects in the
knee joint - A golden standard? J Clin Orthop Trauma 7: 145-152.
12. Mistry
H, Connock M, Pink J, Shyangdan D, Clar C, et al. (2017) Autologous chondrocyte
implantation in the knee: Systematic review and economic evaluation. Health
Technol Assess 21: 1-294.
13. Rosenbaum
AJ, Grande DA, Dines JS (2008) The use of mesenchymal stem cells in tissue engineering.
Organogenesis 4: 23-27.
14. Kim
N, Cho SG (2013) Clinical applications of mesenchymal stem cells. Korean J
Intern Med 28: 387-402.
15. Ding
DC, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transplant 20: 5-14.
16. Dzobo
K, Turnley T, Wishart A, Rowe A, Kallmeyer K, et al. (2016) Fibroblast-derived
extracellular matrix induces chondrogenic differentiation in human
adipose-derived mesenchymal stromal/stem cells in vitro. Int J Mol Sci 17: 1259.
17. Frese
L, Dijkman PE, Hoerstrup SP (2016) Adipose Tissue-derived stem cells in
regenerative medicine. Transfus Med Hemother 43: 268-274.
18. Heo
JS, Choi Y, Kim H-S, Kim HO (2016) Comparison of molecular profiles of human
mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta
and adipose tissue. Int J Mol Med 37: 115-125.
19. Kenry,
Lee WC, Loh KP, Lim CT (2018) When stem cells meet graphene: Opportunities and
challenges in regenerative medicine. Biomaterials 155: 236-250.
20. Tiwari
P, Kumar B, Kaur M, Kaur G, Kaur H (2011) Phytochemical screening and
extraction: A review. Int Pharm Sci 1: 98-106.
21. Khan
WS, Adesida AB, Tew SR, Longo UG, Hardingham TE (2012) Fat pad‐derived
mesenchymal stem cells as a potential source for cell‐based adipose tissue
repair strategies. Cell Prolif 45: 111-120.
22. Razwinani
M, Tshikalange TE, Motaung SCKM (2014) Antimicrobial and anti-inflammatory
activities of Pleurostylia capensis
Turcz (Loes) (celastraceae). Afr J Tradit Complement Altern Med 11: 452-457.
23. Tang
QO, Shakib K, Heliotis M, Tsiridis E, Mantalaris A, et al. (2009) TGF-β3: A
potential biological therapy for enhancing chondrogenesis. Expert Opi Biol Ther
9: 689-701.
24. Hyllested
JL, Veje K, Ostergaard K (2002) Histochemical studies of the extracellular
matrix of human articular cartilage - A review. Osteoarthritis and Cartilage
10: 333-343.
25. Walum
E (1998) Acute oral toxicity. Environ Health Perspect 106: 497-503.
26. Andrades
JA, Motaung SC, Jiménez-Palomo P, Claros S, López-Puerta JM, et al. (2012)
Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal
stem/progenitor cells by transforming growth factor-β1 and bone morphogenetic
protein-7. Arthritis Res Ther 14: R72.
27. Madeira
C, Santhagunam A, Salgueiro JB, Cabral JMS (2015) Advanced cell therapies for
articular cartilage regeneration. Trends Biotechnol 33: 35-42.
28. Armiento
AR, Stoddart MJ, Alini M, Eglin D (2018) Biomaterials for articular cartilage
tissue engineering: Learning from biology. Acta Biomater 65: 1-20.
29. Rambani
R, Venkatesh R (2014) Current concepts in articular cartilage repair. J Arthroscopy
Joint Surg 1: 59-65.
30. Chan
GKY, Kleinheinz TL, Peterson D, Moffat JG (2013) A simple high-content cell
cycle assay reveals frequent discrepancies between cell number and ATP and MTS
proliferation assays. PLoS One 8: e63583.
31. Jones
LJ, Gray M, Yue ST, Haugland RP, Singer VL (2001) Sensitive determination of
cell number using the CyQUANT cell proliferation assay. J Immunol Methods 254:
85-98.
32. Wang
P, Henning SM, Heber D (2010) Limitations of MTT and MTS-based assays for
measurement of antiproliferative activity of green tea polyphenols. PLoS one 5:
e10202.
33. Hoben
G, Willard PV, Athanasiou K (2008) Fibrochondrogenesis of hESCs: Growth factor
combinations and co-cultures. Stem Cells Dev 18: 283-292.
34. Shambaugh
J, Elmer W (1980) Analysis of glycosaminoglycans during chondrogenesis of
normal and brachypod mouse limb mesenchyme. J Embryol Exp Morphol 56: 225-238.
35. Karsdal
MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, et al. (20170 The good and
the bad collagens of fibrosis - Their role in signaling and organ function. Adv
Drug Deliv Rev 121: 43-56.
36. Iozzo
RV, Schaefer L (2015) Proteoglycan form and function: A comprehensive
nomenclature of proteoglycans. Matrix Biol 42: 11-55.
37. Theocharis
AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix
structure. Adv Drug Deliv Rev 97: 4-27.
38. Eyre
DR, Apon S, Wu JJ, Ericsson LH, Walsh KA (1987) Collagen type IX: Evidence for
covalent linkages to type II collagen in cartilage. FEBS Lett 220: 337-341.
39. Symon
A, Harley V (2017) SOX9: A genomic view of tissue specific expression and
action. Int J Biochem Cell Biol 87: 18-22.
40. Zhang
M, Lu Q, Miller AH, Barnthouse NC, Wang J (2016) Dynamic epigenetic mechanisms
regulate age-dependent SOX9 expression in mouse articular cartilage. Int J
Biochem Cell Biol 72: 125-134.
41. Van
Weeren PR (2016) General Anatomy and physiology of joints. In: Joint Disease in
the Horse (2nd Edn). Edinburgh: WB Saunders, pp: 1-24.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Diabetes (ISSN: 2644-3031)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)