794
Views & Citations10
Likes & Shares
Human African trypanosomiasis (HAT) or sleeping sickness, is caused by Trypanosoma brucei gambiense, which is a chronic form of the disease present in western and central Africa and by Trypanosoma brucei rhodesiense, which is an acute disease located in eastern and southern Africa.
Objective: To evaluate the microscopical, serological (CATT; CIATT) and PCR.
Methods: A population of 200 serum samples from 3 displaced health centre around Khartoum state were examined using different techniques blood films, CATT, CIATT test and PCR on whole blood and similar analysis in their cross reactivity which resulted in the detection of the false positive cases of sleeping sickness from patients with malaria, leishmaniasis, Filarial (W. bancrofite) and negative cases show in Toxoplasma gondii and hydatid disease from non-endemic areas of sleeping sickness.
Results: Showed that from 200 serum samples there were 121 male and 79 female detected (17.35% and 24.05%, respectively) and by CIATT detected 7.0% and 6.0%, the result show more positive cases by CATT than CIATT in displaced people in Khartoum state. However parasitological examination did not show the present of the parasites. But in PCR test from 37 blood samples there were 4 cases positive, 10.8%.
Conclusion: By serological analysis the CATT shows more positive cases than CIATT and can be used interchangeably with the old format of the CATT test. It is highly suitable for use in peripheral health facilities in HAT-endemic countries.
INTRODUCTION
African trypanosomiasis; also known as sleeping sickness, is an insect-borne parasitic disease of humans and other animals. Is a deadly disease caused by subspecies of Trypanosoma brucei (Protozoa, Kinetoplastida)—T.b. gambiense and T.b. rhodesiense—transmitted to humans through the bite of insect vectors of the genus Glossina (tse-tse flies) [1], Checchi [2]. There are two types that infect humans, Trypanosoma brucei gambiense (TbG) and Trypanosoma brucei rhodesiense (TbR). TbG causes over 98% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas. Initially, in the first stage of the disease, there are fevers, headaches, itchiness and joint pains. This begins one to three weeks after the bite. Weeks to months later the second stage begins with confusion, poor coordination, numbness and trouble sleeping [3]. Diagnosis is via finding the parasite in a blood smear or in the fluid of a lymph node [3]. A lumbar puncture is often needed to tell the difference between first and second stage disease [3].
Preeminent in this discovery was David Bruce, who, while working in Zululand on a wasting disease of cattle known as nagana, identified trypanosomes in the blood of affected cattle [4,5]. He then established experimentally that healthy game animals were host reservoirs of the disease, which was transmitted by the bite of the tsetse fly to domestic animals, which then became ill [5]. In 1899 the causative parasite was identified as Trypanosoma brucei and in 1902 Everett Dutton first identified, in a European patient, a subspecies of trypanosomes called Trypanosoma brucei gambiense [5] that is now recognized as the cause of West African sleeping sickness. In 1903, Aldo Castellani, working with Bruce, identified trypanosomes in the blood and cerebrospinal fluid (CSF) of a patient with sleeping sickness [5] and in 1910 Stephens and Fantham first described Trypanosoma brucei rhodesiense [5], which is now recognized as causing East African sleeping sickness. There are characteristic differences between the biology and clinical features of T.b. gambiense disease and T.b. rhodesiense disease, probably due to a greater adaptation of the gambiense parasite to humans.
GEOGRAPHICAL DISTRIBUTION
African trypanosomiasis is confined to tropical Africa between latitudes 15°N and 20°S or from north of South Africa to south of Algeria, Libya and Egypt [6]. The prevalence of African trypanosomiasis outside this area varies by country and region. In 2005, major outbreaks occurred in Angola, the Democratic Republic of Congo, and Sudan. In the Central African Republic, Chad, Congo, Côte d’Ivoire, Guinea, Malawi, Uganda, and Tanzania, African trypanosomiasis remains a major public health problem [7-9].
Human African trypanosomiasis (HAT) is a neglected tropical disease that occurs in sub-Saharan Africa, within the distributional limits of the tsetse fly vector. Two forms of the disease exist. The slow-progressing form, caused by Trypanosoma brucei gambiense, is found in Western and Central Africa. The faster progressing form, caused by T.b. rhodesiense, is found in Eastern and Southern Africa [8]. Foci due to T.b. gambiense have been described in the Greater Equatoria Region bordering the Central African Republic [10], Democratic Republic of the Congo and Uganda. HAT caused by T.b. rhodesiense has been reported from areas of Jonglei state (Akobo County) bordering Gambella in Ethiopia [11]; although since 1984 no HAT cases have been reported from either Gambella or Jonglei. In South Sudan, the main vector of the disease is Glossina fuscipes, but G. tachinoides, G. pallidipes and G. morsitans have also been found in the Greater Equatoria Region [12,13].
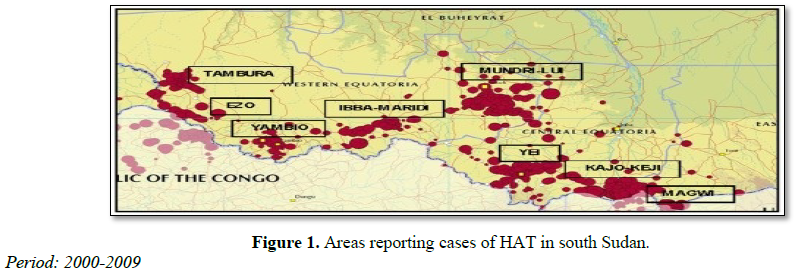
Nine counties in Equatoria are endemic for HAT: Tambura, Ezo, Yambio, Maridi, Ibba, Mundri, Yei, Kajo Keji and Magwi, representing a total population of 1.8 million according to the 2008 census (Figure 1). The 15,754 new cases reported from 692 locations between 2000 and 2009 have been mapped, and detailed epidemiological information on the disease was provided down to the village level. Eight hospitals and five primary health care centers had the capacity for diagnosis and treatment at one time or another between 2000 and 2010, covering all the HAT-endemic counties.
EPIDEMIOLOGY
The epidemiology of Gambian trypanosomiasis is reviewed in detail. The long duration of infection in human hosts with cycles of intermittent parasitemia, the vectors' feeding habits and the intensity of human-fly contact are the major determinants of the dynamics of transmission of this parasite. The development of immunity may lead to a reduction in the fraction of the population that is susceptible to infection and the burning out of epidemics after 20 to 30 years. So far, the acquired immune deficiency syndrome pandemic has had no impact on the epidemiology of Gambian trypanosomiasis. A brief review of the epidemiology of Rhodesian trypanosomiasis highlights the differences from Gambian trypanosomiasis that, to some extent, explain its lower propensity to cause epidemics: it is a more aggressive disease that rapidly kills its human host, and its transmission involves mostly domestic and game animals, humans being in most circumstances an accidental host. The various methods and strategies for the surveillance and control of both diseases are reviewed [14,15].
TRANSMISSION
The trypanosomes causing HAT are classically transmitted by the bite of blood sucking tsetse flies (Diptera, genus Glossina). T.b. gambiense can also be transmitted congenitally [16]. Other routes of transmission are possible but poorly documented and considered extremely rare (sexual, laboratory accidents, blood transfusion, organ transplantation [17]. The principal mode of transmission of HAT is vector-borne by tse-tse fly. The tse-tse fly is considered as a cyclical vector as the transmission requires a transformation process of the parasite in the fly. The disease is mostly transmitted through the bite of an infected tsetse fly but there are other ways in which people are infected. In the case of high parasitemias, the mechanical transmission of the infection by tsetse flies could be possible but very rare and it has already been described for T.b. rhodesiense [18].
PATHOPHYSIOLOGY AND ETIOLOGY
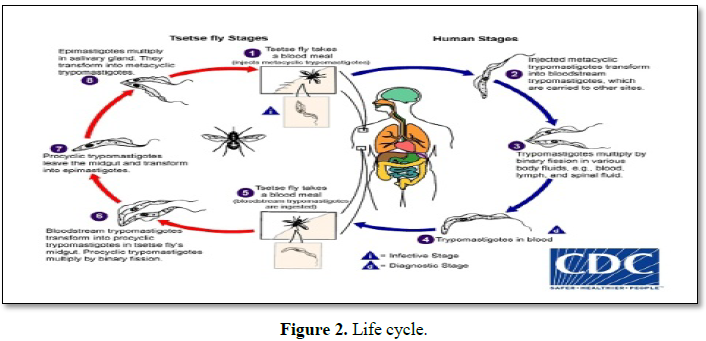
When tsetse flies bite an infected mammalian host, they ingest trypanosomes with the host's blood. After several differentiation steps in the fly, trypanosomes reach the salivary glands. With every subsequent bite, the infected tsetse fly injects trypanosomes with the saliva (Figure 2) [19].
During a blood meal on the mammalian host, an infected tsetse fly (genus Glossina) injects metacyclic trypomastigotes into skin tissue. The parasites enter the lymphatic system and pass into the bloodstream Inside the host, they transform into bloodstream trypomastigotes, are carried to other sites throughout the body, reach other blood fluids (e.g. lymph, spinal fluid) and continue the replication by binary fission, The entire life cycle of African Trypanosomes is represented by extracellular stages. The tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host. In the fly’s midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission, leave the midgut and transform into epimastigotes. The epimastigotes reach the fly’s salivary glands and continue multiplication by binary fission [8]; The cycle in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma brucei gambiense, but this species can also be found in animals. Wild game animals are the main reservoir of T.b. rhodesiense.
At the inoculation site, because of the localized inflammatory reaction, a local chancre may form, which spontaneously resolves within days to weeks. Trypanosomes multiply and spread to the lymphatic system and bloodstream. In the bloodstream, the surface of trypanosomes is entirely covered with variant surface glycoprotein (VSG), which forms a coat of identical proteins. The host mounts an effective antibody response to the VSG, resulting in opsonization (marking of the antigen for destruction by phagocytes) and parasite clearance. Trypanosomes are capable of evading this immune response by antigenic variation: they spontaneously switch their VSG coat, which is then no longer recognized by existing antibodies [20-27].
Eventually, the trypanosomes cross the blood-brain barrier and enter the CNS. They cause extensive meningoencephalitis, infiltration of white matter with inflammatory cells, perivascular cuffing, and astrocyte and macrophage stimulation. The site of invasion and the inflammatory reaction determine the appearance and type of neuropsychiatric symptoms. Brain invasion is accompanied by a strong intrathecal immunoglobulin response [28]. Production of cytokines (IL-10, IL-6) and chemokines (CXCL-8, XCL-10, CXCL-13) in the CNS seems to be related to progression of the disease and severity of the neurologic signs [23-26]. If untreated, patients usually progress to coma, severe organ failure and eventually death [27,28].
SYMPTOMS
Following the bite of the infected fly (both male and female can transmit infection), the parasite multiplies in the lymph and the blood of the person bitten, causing unspecific symptoms and signs such as headaches, fever, weakness, pain in the joints, lymphadenopathy and stiffness. People who become infected may or may not show signs of illness immediately, but over time the parasite crosses the blood-brain barrier and migrates to the central nervous system. Here it causes various neurological changes which include the sleep disorder (hence the name “sleeping sickness”), deep sensory disturbances, abnormal tone and mobility, ataxia, psychiatric disorders, seizures, coma and ultimately death.
In the case of T.b. rhodesiense infections, the disease is acute, lasting from a few weeks to several months while in T.b. gambiense infections the disease is chronic, generally progressing slowly over several years. If left untreated, the infection worsens, and death may occur in several months to several years [22].
DIAGNOSTIC METHODS
Diagnosis of sleeping sickness is based on parasitological laboratory diagnosis blood for wet; thick and thin films stained by Giemsa stain is used for detection and identification even in the hands of well-trained technologist; and serological There are two field tests for diagnosis of the disease, namely Card Agglutination Test for Trypanosomiasis CATT for diagnosis of T.b. gambiense disease; the other is the Card Indirect-Agglutination Antigen. Test for Trypanosomiasis (CIATT) for the diagnosis of T.b. gambiense and T.b. rhodesiense disease PCR examinations. Serological tests Card Agglutination Test for Trypanosomiasis (CATT) The test was carried out as described by Magnus et al. [29]. On the other hand, the sensitivity of the screening test can be improved by adding a Ca2+/Mg2+ chelating agent (i.e., Na-EDTA) to the reaction thus avoiding a complement mediated prozone phenomenon [28,29] Is a fast and simple agglutination assay for detection of T.b. gambiense-specific antibodies in the blood, plasma, or serum of HAT patients. Reported sensitivity of CATT on undiluted blood varies from 68.8% to 100% [22]); Boatin [29], Chappuis [30], Fairlamb [31] and Bu ̈scher [32] reported specificity ranges between 83.5% and 99.3% and reproducibility is good (Kappa coefficient = 0.84) [33]. A preliminary evaluation of the Tryp Tect CIATT showed a high sensitivity compared to other parasitological techniques [34], but the results of subsequent studies raised strong doubts about its specificity [35].
BIOLOGICAL PARAMETERS
Biological blood parameters such as increased sedimentation rate and low hematocrit reflect the systemic chronic inflammation present in HAT patients and are therefore nonspecific. Thrombocytopenia is generally mild or absent, and features of disseminated intravascular coagulopathy are not found [36]. Liver transaminase levels, bilirubin, and renal function tests are usually within normal limits or slightly elevated [37]. Protein measurements usually show decreased albumin and increased immunoglobulin concentrations, especially of IgM [8]. Low serum C3 levels and split C3 products can be found, reflecting complement activation. The following studies may also be considered: Computed tomography (CT) of the head; Magnetic resonance imaging (MRI) of the head and Electroencephalography (EEG).
MATERIALS AND METHODS
Study site
The study was conducted in Khartoum state. It’s well outside the tsetse belt in Sudan.
Sampling
A total of 200 blood samples for serum separation were collected aseptically in anticoagulation and plain container, from the three health centers of displaced people’s camps who originally came from endemic areas in Southern Sudan according to the willingness of the people for sampling (Convenience sampling methods). Around Khartoum state (Elsalam Medical center in central Mandela - Mayo, Dar Elsalam Jabel Awella Medical Health Center and Wed Elbasher Omdurman Medical Health Center) was taken to the National Health Laboratory for examination. After obtaining the informed consent of patients, a questionnaire was filled for each individual and blood samples. The first step is to conduct blood and other tests. A microscope is used to confirm the presence of the agent.
PARASIUTOLOGICAL DIAGNOSIS
Parasitological examination Wet thick and thin blood films. In wet blood films, 5-10 μl of finger prick blood is placed on a slide and examined microscopically (magnification, x400) under a cover slip. Trypanosomes can be seen moving between the erythrocytes (the movement of the surrounding erythrocytes often attracts attention). Despite its very low sensitivity, with a detection limit as high as 10,000 trypanosomes/ml, corresponding to 1 parasite/200 microscope fields, this method is still used in some centers because of its low cost and simplicity.
Thin blood films were prepared on slides, dried and fixed with alcohol and stained with 3% diluted Giemsa’s stain solution for 30 min, washed in buffered distilled water (PH 6.8-7.2). They were left to dry and then examined microscopically. Giemsa- or Field's-stained thin blood films have a similarly low sensitivity.
Examination of 20 μl of stained blood in a thick blood film slightly improves sensitivity, with a detection threshold of around 5,000 trypanosomes/ml. It is the technique of choice for blood examination only when no centrifuge is available [37], although it is quite time-consuming (10-20 min/slide) and requires expertise to recognize the parasite, which is frequently deformed in this preparation. Apart from trypanosomes, other parasites such as microfilaria and Plasmodium can be detected.
Serological diagnosis
Reagents: CATT reagent: Stabilized freeze saline solution of fixed by formaldehyde-fixed and Coomassie-blue-stained bloodstream-form of T.b. gambiense. The antigen suspension was divided into two equal volumes. One volume was processed with the standard CATT lyophilisation medium, divided over separate vials each containing 1.05 ml and freeze dried. For the other a different lyophilisation medium was used: the volume was divided over separate vials each containing 0.375 ml and freeze dried. Each vial is reconstituted with 2.5 ml of CATT buffer and allows the performance of at least 50 tests. The freeze dried antigen and control sera should be kept at between 4-8°С. The shelf-life of antigen, control sera and buffer is 1 year, when refrigerated at below 10°С. The reconstituted reagents can be used for up to 7 days when stored at 4-8°С or up to 8 h under field non-refrigerated conditions. The antigens suspension must not be frozen. Each vial of the reagent sufficient for 50 tests.
CATT buffer: Phosphate buffered saline solution (PBS, PH 7.2) for reconstitution of CATT preservative: sodium azide (0.1%).
Positive control serum: To be reconstituted with 0.5ml of CATT buffer.
Negative control serum: To be reconstituted with 0.5ml of CATT buffer.
CATT procedure
One drop (about 45 µl) well homogenized CATT reagent was placed on each area of the card 5 µl serum spread the reaction mixture using a stirring rod.
Mix well with stirring rod and left for 5 min on rotator.
Reading results after the 5 min depended on the intensity of agglutination.
Card indirect agglutination test for trypanosomiasis (CIATT)
Specimen: The test is best performed on serum.
CIATT reagent: Tris buffer
Positive control serum
Negative control serum
Principle of the test
The CIATT reagent is a suspension of latex particles which have been sensitized with a specific monoclonal antibody against an invariant, internal antigen which is common to both T.b. gambiense and T.b. rhodesiense following the procedure described by Chappuis et al. [38].
IHA (CIATT) method
Procedure:
Introduce serum - 5 µl
Add CIATT (IHA) reagent - 100 µl
Mix well with rotator or by hand for 2-3 h after that read this is quantitative method but qualitative method is in micro titration plates of polystyrene with v-bottom wells diluted the serum and positive control 1/7 (1:8) respectively in tris buffer PH 8.0 (50 µl serum: 350 µl buffer) into well1 A – Hand 50 µl of tris buffer solution into the remaining wells with the exception of negative control diluted 1:16 into well A12.
PCR: The polymerase chain reaction (PCR) has been proposed for diagnosis, staging and post-treatment follow-up of sleeping sickness but no large-scale clinical evaluations of its diagnostic accuracy have taken place yet.
Other investigation tests for cross-reaction:
· Visceral leishmaniasis 30 blood samples were collected from Gaderif hospital.
· ICT rapid strip test for detection of antibody of visceral leishmaniasis (blood; plasma; or serum).
· Serum of positive malaria parasites also from Gaderif hospital.
· ICT for lymphatic filariasis the 200 samples gave smooth reaction (no agglutination).
· Toxoplasmosis 15 serum samples.
· Hydatid disease 15 serum samples.
RESULTS
Microscopic examination
All the 200 blood films were negative by wet, thick and thin for detection of trypomastigote.
Serological examination
Using CATT on 200 serum samples from the three health centers of displaced people’s camps in Khartoum state, who originated from endemic areas in southern Sudan, showed 40 positive cases (20.0%) and 14 positive cases (7.0%) respectively. However, a microscopic examinations using wet, thick and thin blood film gave negative results in all samples (Table 1). Positive cases were found statistically to be higher in males than females (7.0%, 6.0%, respectively (P=0.840).
A comparison between CATT and CIATT for diagnosis of sleeping sickness was made on 200 serum samples from displaced people’s camps the result showed 40 positive cases for CATT (20.0%) but only 14 positive cases were detected by CIATT (7.0%) (The intensity of reaction is given in Tables 2 and 3. Similar cross-reaction in positive serum from trypanosomiasis-free areas was examined serologically. 30 patients from Gaderif state with malaria 30 (3.0%) and serum from 30 patients with leishmaniasis from the ward of leishmania from Gaderif state (6.0%), W. bancrofite 200 cases showed (2.5%). 15 cases of toxoplasmosis the prevalence rate -(0.0%) and the last test was hydatidosis 15 positive showed prevalence rate (0.0%) from statistically CATT and CIATT were found similar in the detection of the false positive cases from patients with malaria and leishmaniasis from non-endemic areas, on the other hand we found negative results in Toxoplasma gondii and hydatid disease 0.0%.
Card agglutination test for trypanosomiasis (CATT)
The result was assessed as following:-
1. Positive reaction +++ : (very strong agglutination)
2. Positive reaction ++ : (strong agglutination)
3. Positive reaction + : (still good agglutination)
4. Negative reaction - : (absence agglutination)
Mandella Health Centre
120 blood samples were collected for serological screening and the titre from these 120 samples showed 18 positive samples. The prevalence rate was 15%. The titre (1:10 showed 9.2%), (1:20 showed 3.3%), 1:40 showed 2.5%). All blood stained by Giemsa stain were negative, and dried blood samples on filter paper was done by CATT reagent gave 19 positive samples with the prevalence rate 15.8% respectively this requires a smaller quantity of reagents than that needed for the CATT on serum samples and the cost is therefore reduced the results seem less reliable than for the CATT on serum, but this depends on the technique used. Large scale evaluation is needed to assess the reliability of CATT on dried blood samples in different epidemiological situation. The serial dilution of the blood samples in 120 blood samples there were 19 blood samples (15.8%) (1:10 show 10.0%), (1:20 show 3.3%), 1:40 show 2.5%).
Dar Elsalam Health Centre at Jabel Aula
Serological CATT in Dar Elsa am the second health centre in Jabel Aula where 73 cases showed 22 positive samples at 5.8% in serial dilution (1:10 show 13.6%), (1:20 show 10.9%) and 1:40 show 5.4%). However no variation observed in intensity of reaction between Mandella Health Centre and Dar Elsalam Health Centre at Jabel Aula.
Wad Elbasher Health Centre at Ombada
The Health Centre in Omdurman at Ombada Wad Elbasher 7 cases were negative which were serologically screened and blood film for Trypanosomiasis were examined.
CATT in filter paper
From a total of 200 blood samples there were 37 cases positive (prevalence rate of 18.5%) as seen from the results the T.b. gambiense dried samples on filter papers be tested by CATT. This requires a smaller quantity of reagents than that needed for CATT on serum samples and the cost is therefore reduced the results seem less reliable than for the CATT on serum, but this depends on technique used (Figures 3 and 4).
CIATT
CIATT was done by mixing 25 μl of serum or plasma with 25 μl of CIATT reagent and shaken on the rotator for 5 min [39].
The result is to be read against a white background or with the aid of a mirror.
Complete agglutination of the cell: Positive
Agglutination with the ring formation: Weekly positive
Sedimentation cell: Negative
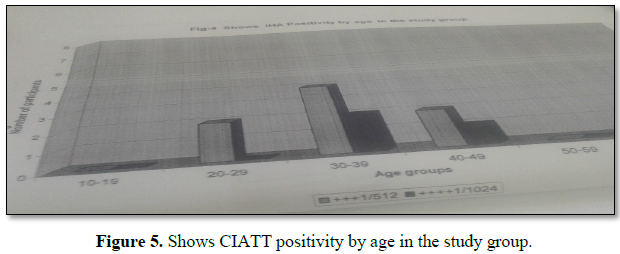
From 200 blood samples 14 positive results were obtained (prevalence rate of (7.0%) as seen from the results there were variations between positive cases detected by CATT and CIATT in displace people in Khartoum state. CATT detected more positive cases than CIATT (20.0%, 14.0% respectively). However parasitological examination did not show the presence of parasites. There was dilution of the reagent 1+7 (1:8) from 1:2, till 1:512 go on titer until 1:4096 is the significant titer before is cu-off (1:512), there were 14 samples (Figure 5 and Table 4).
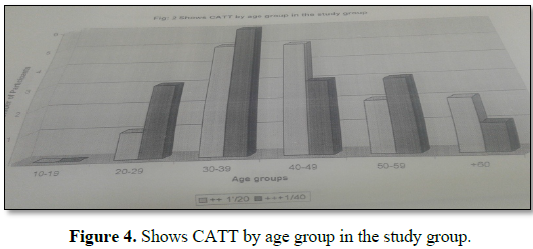
Prevalence rate by sex was found to be higher in males compared to females; in males (60.5%) and in females (35.0%). More positive cases were detected by serological screening in age group 30-39 years (33.0%) than in other groups 10-29 years and from 40-60 years.
Other investigation tests for cross-reaction:-
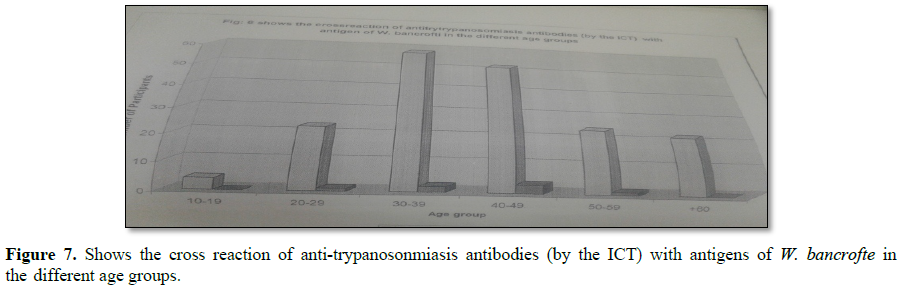
A simple and rapid test, the card indirect agglutination trypanosomiasis test (CIATT) is described, for detecting circulating antigens in persons suffering from Trypanosoma brucei gambiense and T.b. rhodesiense infection by latex agglutination. As sera from 30 (3.0%) patients with malaria, 30 (6.0%) with visceral leishmaniasis, 15 with toxoplasmosis (0.0%), 200 (2.5%) with filariasis and 15 (0.0%) with hydatid disease, from trypanosomiasis-free areas, gave negative results. without microscopically detectable parasitemia. This study showed that CIATT is a useful test for rapid diagnosis of both T.b. gambiense and T.b. rhodesiense infections (Figures 6 and 7 and Table 5).
DISCUSSION
Human African Trypanosomiasis or sleeping sickness has been a public health problem in Sudan throughout most of the 20th century. Endemic foci of T.b. gambiense are found in southern Sudan. The disease in Sudan has been mainly of the Gambian type transmitted mostly by Glossina fuscipes [40]. The latest civil unrest has resulted in a massive resurgence of the disease in the republic of southern Sudan, in the provinces of western and eastern Equatoria. When a bilateral Belgian-Sudanese trypanosomiasis treatment and control programme was implemented. This effort brought the disease under relatively good control within a few years. However trypanosomiasis programme collapsed in 1990 during the Civil war. Sleeping sickness treatment in Southern Sudan remained available at a single site the La Rangu Hospital in Yambio country. As treatment drugs were in limited supply staff members were unpaid and transport to the hospital was not available, there was virtually no active case detection in Southern Sudan for more than 8 years.
High seroprevalence of sleeping sickness using CATT was recorded in Khartoum state. However, Khartoum state is known as a non-endemic area. This could be explained by the population movement from endemic areas. The seroprevalence was found higher in males than in females (9 (7.0%) and 5 (6.0%), respectively). It is possible the males are in more frequent contact with the tsetse infested areas where they come for hunting, fishing, farming and herding (Table 2). As seen from the results there was a variation between positive cases detected by CATT and CIATT (20.0% and 7.0%, respectively). This could be attributed to the fact that CATT was developed to detect antibodies directed against a specific commonly occurring variable antigen of T.b. gambiense. While, CIATT for diagnosis of both T.b. gambiense and T.b. rhodesiense is based on the detection of the specific circulating trypanosomal antigens in blood [41]. However, CATT has a number of operational limitations that hinder its large-scale implementation, especially in basic health facilities in remote areas, including the need for specialized equipment, electricity and refrigeration. The sensitivity and specificity of CATT have also been reported to be sub-optimal in a number of settings [42]. Moreover, recent report has suggested that CATT is not effective in all T.b. gambiense endemic foci [43]. Individuals who react positively in the CATT or CIATT and then gave a negative result for parasitological methods. This may be due to the variation between the regions as well as a number of positive tests are not confirmed by the presence of parasites in the blood, lymph node aspirates or cerebrospinal fluid. The finding was previously confirmed by who explained that either some of the sero-positive patients were indeed infected but their parasitemia is too low to be detected, or they were false positives. Furthermore, due to the lack of sensitivity and specificity of serological tests was developed real-time PCR assay and he indicated that it can be considered as a rapid and sensitive method suitable for the detection of T. brucei in human blood samples in routine clinical laboratory practice [44]. The false positive cases detected by CATT and CIATT in patients with malaria and leishmaniasis from non-endemic areas of sleeping sickness however, high sensitivity and specificity were recorded by different authors [27,45]. The disagreement is most probably due to the cross-reaction. More investigation is needed in order to establish possible interaction between both malaria and leishmaniasis with human African trypanosomiasis, but in Toxoplasma gondii and hydatid disease give negative result [14,18,46-50].
Polymerase chain reaction (PCR) test evaluated on DNA extracted from 37 positive blood samples which were collected from Dar Elsalam health center in Jabal Awlia by CATT samples were negative when tested parasitologically. In the 37 blood samples there were 4 cases positive by PCR. The prevalence rate was l0.8%. Since there is evidence that some of the unconfirmed CATT positive individuals are indeed infected lowering the detection limit of the PCR method [9,51-56].
CONCLUSION
In conclusion, sleeping sickness remains a major public health concern in south Sudan. Care must be taken in interpretation of serological tests. Based on that parasitological confirmation and clinical stage of the disease are needed before commencement of treatment [40,56-60].
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTION
AAMA was collected blood from displaces area, examined microscopic for the trypomastigote and serological analysis. MEH analyzed the final manuscript; MEI is director of the lab was helped with her experience.
ACKNOWLEDGEMENT
The authors would like to thank the members of the staff of Trypanosomiasis for their participation in fruitful discussions, which have been considered for this paper. Special acknowledgment to Muntser Eltyeb and Mohammad Eltyeb for the critical reading of the document.
1. Blum J, Schmid C, Burri C (2006) Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop 97: 55-64.
2. Checchi F, Filipe JA, Haydon DT, Chandramohan D, Chappuis F (2008) Estimates of the duration of the early and late stage of gambiense sleeping sickness. BMC Infect Dis 8: 16.
3. Kennedy (2013) Clinical features, diagnosis and treatment of human African trypanosomiasis (sleeping sickness).
4. Vickerman K (1997) Landmarks in trypanosome research. In Trypanosomiasis and leishmaniasis, Hide G, Mottram JC, Coombs GH and Holmes PH, editors. Cab International, Oxford: United Kingdom, pp: 1-37.
5. Williams BI (1996) African trypanosomiasis. In The Wellcome Trust illustrated history of tropical diseases. F.E.A.G. Cox, editor. The Wellcome Trust: London, United Kingdom, pp: 178-191.
6. Franco JR, Simarro PP, Diarra A, Jannin JG (2014) Epidemiology of human African trypanosomiasis. Clin Epidemiol 6: 257-275.
7. Simarro PP, Cecchi G, Franco JR, Paone M, Fèvre EM, et al. (2011) Risk for human African trypanosomiasis, central Africa. Emerg Infect 2011: 2322-2324.
8. Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A (2010) The atlas of human African trypanosomiasis: A contribution to global mapping of neglected tropical diseases. Int J Health Geogr 9: 57.
9. Pansaerts R, Van Meirvenne N, Magnus E, Verhelst L (1998) Increased sensitivity of the card agglutination test CATT/Trypanosoma brucei gambiense by inhibition of complement. Acta Trop 70: 349-354.
10. Ruiz Postigo JA, Franco JR, Simarro PP, Bassets G, Nangouma A (2001) Cost of a national program to control human African trypanosomiasis in the high Mbomou region, Central African Republic. Med Trop 61: 422-424.
11. Baker JR, McConnell E, Kent DC, Hady J (1970) Human trypanosomiasis in Ethiopia: Ecology of Illubabor province and epidemiology in the Baro river area. Trans R Soc Trop Med Hyg 64: 523-530.
12. Pépin J, Méda HA (2001) The epidemiology and control of human African trypanosomiasis. Adv Parasitol 49: 71-132.
13. Snow WF, Declercq J, van Nieuwenhove S (1991) Watering sites in Glossina fuscipes habitat as the major foci for the transmission of Gambiense sleeping sickness in an endemic area of southern Sudan. Ann Soc Belg Med Trop 71: 27-38.
14. Pépin J, Khonde N, Maiso F (2000) Short-course eflornithine in Gambian trypanosomiasis: A multicentre randomized controlled trial. Bull World Health Org 78: 1284-1295.
15. Atouguia JLM, Kennedy PGE (2000) Neurological aspects of human African trypanosomiasis. In Infectious diseases of the nervous system, Davis LE and Kennedy PGE, edrs. Butterworth-Heinemann, Oxford: United Kingdom, pp: 321-372.
16. Lindner AK, Priotto G (2010) The unknown risk of vertical transmission in sleeping sickness - A literature review. PLoS Negl Trop Dis 4: e783.
17. Rocha G, Martins A, Gama G, Brandão F, Atouguia J (2004) Possible cases of sexual and congenital transmission of sleeping sickness. Lancet 363: 247.
18. Roberts LW, Wellde BT, Reardon MJ, Onyango FK (1989) Mechanical transmission of Trypanosoma brucei rhodesiense by Glossina morsitans morsitans (Diptera: Glossinidae). Ann Trop Med Parasitol 83: 127-131.
19. Vickerman K (1985) Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull 41: 105-114.
20. Pays E, Nolan DP (1998) Expression and function of surface proteins in Trypanosoma brucei. Mol Biochem Parasitol 1998: 3-36.
21. Lejon V, Reiber H, Legros D (2003) Intrathecal immune response pattern for improved diagnosis of central nervous system involvement in trypanosomiasis. J Infect 187: 1475-1483.
22. Bisser S, Lejon V, Preux PM, Bouteille B, Stanghellini A, et al. (2002) Blood-cerebrospinal fluid barrier and in trathecal immunoglobulins compared to field diagnosis of central nervous system involvement in sleeping sickness. J Neurol Sci 193: 127-135.
23. Magnus E, Lejon V, Bayon D, Buyse D, Simarro P, et al. (2002) Evaluation of an EDTA version of CATT/Trypanosoma brucei gambiense for serologiocal screening of human blood samples. Acta Tropica 81/1: 7-12.
24. MacLean L, Odiit M, Sternberg JM (2006) Intrathecal cytokine responses in Trypanosoma brucei rhodesiense sleeping sickness patients. Trans R Soc Trop Med Hyg 100: 270-275.
25. Courtioux B, Pervieux L, Gedeao V (2009) Increased CXCL-13 levels in human African trypanosomiasis meningoencephalitis. Trop Med Int Health 14: 529-534.
26. Hainard A, Tiberti N, Robin X (2009) A combined CXCL10, CXCL8 and H-FABP panel for the staging of human African trypanosomiasis patients. PLoS Negl Trop Dis 3: e459.
27. Sternberg J (2004) Human African trypanosomiasis: Clinical presentation and immune response. Parasite Immunol 26: 469-476.
28. Kennedy PG (2008) The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurol 64: 116-126.
29. Boatin BA, Wyatt GB, Wurapa FK, Bulsara MK (1986) Use of symptoms and signs for diagnosis of Trypanosoma brucei rhodesiense trypanosomiasis by rural health personnel. Bull World Health Organ 64: 389-395.
30. Chappuis F, Pittet A, Bovier PA, Adams K, Godineau V, et al. (2002) Field evaluation of the CATT/Trypanosoma brucei gambiense on blood-impregnated filter papers for diagnosis of human African trypanosomiasis in southern Sudan. Trop Med Int Health 7: 942-948.
31. Borst P, Fairlamb AH (1998) Surface receptors and transporters of Trypanosoma brucei. Annu Rev Microbiol 52: 745-778.
32. Bu ̈scher P, Lejon V, Magnus E, Van Meirvenne N (1999) Improved latex agglutination test for detection of antibodies in serum and cerebro-spinal fluid of Trypanosoma brucei gambiense infected patients. Acta Trop 73: 11-20.
33. Burri C, Brun R (2003) Human African trypanosomiasis. In: Cook GC and Alimuddin Z (ed.), Manson’s tropical diseases. 21st Edn. Elsevier Science: Edinburgh, United Kingdom.
34. Nantulya VM (1997) TrypTect CIATT—card indirect agglutination trypanosomiases test for diagnosis of Trypanosoma brucei gambiense and T.b. rhodesiense infections. Trans R Soc Trop Med Hyg 91: 551-553.
35. Asonganyi T, Doua F, Kibona SN, Nyasulu YM, Masake R, et al. (1998) A multi-centre evaluation of the card indirect agglutination test for trypanosomiasis (TrypTect CIATT). Ann Trop Med Parasitol 92: 837-844.
36. Henry MC, Kageruka P, Ruppol JF, Bruneel H, Claes Y (1981) Evaluation of field diagnosis of trypanosomiasis caused by Trypanosoma brucei gambiense. Ann Soc Belg Med Trop 61: 79-92.
37. Moore A, Richer M (2001) Re-emergence of epidemic sleeping sickness in southern Sudan. Trop Med Int Health 6: 342-347.
38. Chappuis F, Loutan L, Simarro P, Lejon V, Buscher P (2005) Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev 18: 133-146.
39. Komba (1992) Multicentre evaluation of an antigen-detection ELISA for the diagnosis of Trypanosoma brucei rhodesiense sleeping sickness. Bull World Health Organ 70: 57-61.
40. Mohammed YO, El Malik KH, Mohamed-Ahmed MM, Intisar ER (2010) Factors influencing the sero-prevalence of Trypanosoma brucei gambiense sleeping sickness in Juba County, Central Equatoria State, Southern Sudan. J Pub Health Epidemiol 2: 100-108.
41. Dukes P, Gibson WC, Gashumba JK, Hudson KM, Bromidge TJ, et al. (1992) Absence of LiT at 1.3 (CATT antigen) gene in Trypanosoma brucei gambiense stock from Cameroon. Acta Trop 51: 123-134.
42. Becker S, Franco JR, Simaro PP, Stich A, Abel PM, et al. (2004) Real time PCR for detection of Trypanosoma brucei in human blood samples. Diagn Microbiol Infect Dis 5: 193-199.
43. World Health Organization (2013) Control and Surveillance of Human African Trypanosomiasis Report of a WHO Expert Committee WHO Technical Report Series 984. Geneva, Switzerland: World Health Organization.
44. World Health Organization (2000) World Health Report 2000 Health Systems Improving Performance, Geneva.
45. WHO Media Centre (2014) Fact sheet N°259: Trypanosomiasis, Human African (sleeping sickness). World Health Organization.
46. Tong J, Valverde O, Mahoudeau C, Yun O, Chappuis F (2011) Challenges of controlling sleeping sickness in areas of violent conflict: Experience in the Democratic Republic of Congo. Confl Health 5: 7.
47. Truc P, Lando A, Penchenier L, Vatunga G, Josenando T, et al. (2012) Human African trypanosomiasis in Angola: Clinical observations, treatment and use of PCR for stage determination of early stage of the disease. Trans R Soc Trop Med Hyg 106: 10-14.
48. Rock KS, Torr SJ, Lumbala C, Keeling MJ (2015) Quantitative evaluation of the strategy to eliminate human African trypanosomiasis in the Democratic Republic of Congo. Parasit Vectors 8: 532.
49. Picozzi K, Fèvre EM, Odiit M, Carrington M, Eisler MC, et al. (2005) Sleeping sickness in Uganda: A thin line between two fatal diseases. BMJ 331: 1238-1241.
50. Roberts LW, Wellde BT, Reardon MJ, Onyango FK (1989) Mechanical transmission of Trypanosoma brucei rhodesiense by Glossina morsitans morsitans (Diptera: Glossinidae). Ann Trop Med Parasitol 83: 127-131.
51. Pays E (1999) Antigenic variation in African trypanosomes. In: Dumas M, Bouteille B, Buguet A, eds. Progress in human African trypanosomiasis, sleeping sickness. Paris: Springer, pp: 31-52.
52. Pandey A, Atkins KE, Bucheton B, Camara M, Aksoy S, et al. (2015) Evaluating long-term effectiveness of sleeping sickness control measures in Guinea. Parasit Vectors 8: 550.
53. Mumba D, Bohorquez E, Messina J, Kande V, Taylor SM, et al. (2011) Prevalence of human African trypanosomiasis in the Democratic Republic of the Congo. PLoS Negl Trop 5: e1246.
54. Nantulya VM, Doua F, Molisho S (1992) Diagnosis of Trypanosoma brucei gambiense sleeping sickness using an antigen detection enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg 86: 42-45.
55. Moore A, Richer M, Enrile M, Losio E, Roberts J, et al. (1999) Resurgence of sleeping sickness in Tambura County, Sudan. Am J Trop Med Hyg 61: 315-318.
56. McConnell E, Hutchinson MP, Baker JR (1970) Human trypanosomiasis in Ethiopia: The Gilo River area. Trans R Soc Trop Med Hyg 64: 683-691.
57. Magnus E, Vervoort T, Van Meirvenne N (1978) A card agglutination test with stained trypanosomes (CATT) for the serological diagnosis of Trypanosoma brucei gambiense trypanosomiasis. Ann Soc Belg Med Trop 58: 169-176.
58. Lejon V, Lardon J, Kenis G (2002) Interleukin (IL)-6, IL-8 and IL-10 in serum and CSF of Trypanosoma gambiense sleeping sickness patients before and after treatment. Trans R Soc Trop Med Hyg 96: 329-333.
59. Greenwood BM, Whittle HC (1976) Coagulation studies in Gambian trypanosomiasis. Am J Trop Med Hyg 25: 390-394.
60. Greenwood BM, Whittle HC (1976) Complement activation in patients with Gambian sleeping sickness. Clin Exp Immunol 24: 133-138.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)