693
Views & Citations10
Likes & Shares
INTRODUCTION
Reactive oxygen species (ROS) are considered as
toxic molecules and have been associated with pathological conditions including
cancer, aging and neurodegenerative disorders [1]. During cellular
metabolism, molecular oxygen (O2) is reduced to superoxide which
triggers the generation of other reactive species. Since ROS are highly
reactive and unstable, they can easily interfere with other cellular processes
resulting in protein and DNA destruction, fatty acid oxidation
and synaptic plasticity [2]. Though the body naturally reduces ROS, toxic
effects occur when the destructive effects of ROS surpass the ability of
the body to neutralize the oxidizing species and repair cellular damage [3].
Plants are important sources of bioactive compounds
and have been used in traditional medicine for centuries. The health effects of
plant products result from the presence of components such as alkaloids,
cardiac glycosides, tannins, sterols, triterpenes, anthocyanins [4], flavonoids
[5], and other phenolic compounds [6]. Phenolic compounds have potent
antioxidant potency and have been shown to protect against coronary heart
disease and cancer. Due to the toxic effects of synthetic antioxidants such
as butylhydroxyanisole and butylatedhydroxytoluene [7], the search
and use of natural antioxidants from plant sources have increased.
Calotropis
procera Linn., a wild growing plant of the family
Asclepiadaecae, is known to possess diverse medicinal properties. Crude
extracts of different parts of this plant have been used in traditional medicine
and have been shown to treat ulcer, diabetes [8], inflammation [9] and diseases
of the spleen and liver [10]. However, treating disease with crude plant
extracts may not be completely safe. Some extracts, despite their positive
effects, may also cause severe side effects such as liver damage. To the best
of our knowledge, no study has been done to ascertain the hepatotoxicity
of Calotropis procera leaf extracts after their
antioxidant properties have been determined. Therefore, in this study, we investigated
the antioxidant activity of various extracts of Calotropis procera leaves in
vitro. We then studied the acute toxicity and hepatotoxic effects of the
extracts in albino rats.
MATERIALS AND METHODS
Leaves
Leaves of Calotropis procera were
collected from the University of Cape Coast-Ghana, School of Biological
Sciences botanical garden and authenticated by the herbarium. The leaves were
washed and air-dried for one week.
Preparation of extracts
The dried leaves were ground into powder (177g) and
divided into three equal parts. One part (59.14g) was extracted with 250 mL of
70% methanol (Zayo-Sigma Chemicals Ltd.-Nigeria) using a Soxhlet
extractor for 48 hours. The other parts of the powdered samples were
extracted with either distilled water or ethyl acetate. The extracts were
heated at 60 ºC to expel the solvents and stored at 4ºC
till use.
In vitro Antioxidant Activity
β-carotene bleaching assay
The β-carotene bleaching inhibitory assay was
carried out according to a method reported earlier [11]. Briefly,
β-carotene solution was prepared by dissolving 2 mg of β-carotene (Selleck
Chemicals- South Krea) in 10 mL chloroform (Zayo-Sigma Chemicals Ltd.-Nigeria)
and 1.0 mL of this solution was then pipetted out into a flask containing 20 mg
of linoleic acid (Zayo-Sigma Chemicals Ltd.-Nigeria) and 200 mg of Tween 40
emulsifier (Zayo-Sigma Chemicals Ltd.-Nigeria). One mL of the solution was then
pipetted into a flask and chloroform was completely evaporated using a water
bath at 40 0C. Fifty mL of distilled water was then added.
Aliquots of 5 mL of this emulsion were transferred into a series of tubes
containing various concentrations of the extracts (50-250 μg/mL) or α
tocopherol (control). The absorbance of the fractions and the control were
measured immediately (t=0). The tubes were incubated at 50ºC in a
water bath and the absorbance was measured at 20 min interval at 470 nm till
120 min (t=120) using a spectrophotometer (Bio-Rad SmartSpec 3000 UV/Vis).
Ferric reducing ability
The reducing ability of the leaf extracts were
determined by the potassium ferricyanide-ferricchloride method reported earlier
[12]. One mL of the different dilutions of extracts (50-250 μg/mL) was added to
2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide
(The Science Company, Lakewood). The mixtures were incubated at 50 ºC
for 20 min, after which 2.5 mL trichloroacetic acid (10%) was added. An aliquot
of the mixture (2.5 mL) was taken and mixed with 2.5 mL water and 0.5 mL 1%
FeCl3 (The Science Company, Lakewood). The absorbance at 700 nm
was measured after allowing the solution to stand for 30 min.
Hydrogen peroxide scavenging assay
Hydrogen peroxide solution (2 mM) was prepared with
standard phosphate buffer (pH 7.4). Different concentrations of the leaf
extracts (50-250 μg/mL) in distilled water were added to 0.6 mL of hydrogen
peroxide solution. Absorbance was determined at 230 nm after 10 min against a
blank solution containing phosphate buffer without hydrogen peroxide. The
percentage scavenging activity at different concentrations of the fractions was
determined and compared with the standard, α-tocopherol [13].
Toxicological Studies
Animals
Albino rats (180–220 g) of
either sex were obtained from the animal house, School of Biological Sciences,
University of Cape Coast, Ghana. The rats were housed in proper cages with
adequate lighting and ventilation at an ambient temperature of 28-30ºC. The
rodents were fed with standard rodent diet and with water ad
libitum. Approval from the University of Cape Coast animal ethical committee
for the usage of animals in the experiments was obtained.
Acute toxicity
The rats were randomly
divided into three groups each containing 5 rats. Doses of extract (0.5, 1 and
2g/kg body weight) were given orally, a dose to each group, and the effects
observed. The overall behaviour exhibited by the animals was recorded using a checklist,
which included urinary frequency, defecation, changes in locomotory activity,
convulsion, lacrimation, and salivation, within the first 6 hours after extract
administration. The animals were observed for 48 hours during which time the
number of death were recorded.
Subacute toxicity
Rats of either sex weighing
between 180 and 220g were randomised into four groups each containing 5 rats.
The first, second and third groups were orally given 0.667, 0.33, and 0.167g/kg
body weight respectively of the plant extract daily for 28 consecutive
days while rats in the fourth group (control) were given 1mL
of distilled water daily for 28 consecutive days. After 28 days, blood samples were collected by cardiac puncture
and then centrifuged at 2000g for 10 min to separate the serum for the various
biochemical analyses. Biochemical parameters (serum glutamate
oxaloacete transaminase (AST), serum glutamate pyruvate transaminase (GGT),
serum alkaline phosphatase (ALP), alanine aminotransferase (ALT) and serum
bilirubin were determined on the 29th day after completion of treatment.
Biochemical analysis
The collected blood samples were used for the
analysis of biochemical markers AST [14], ALT [15], SGGT [16], ALP [17],
biluribin [10] levels.
Statistical analysis
The results were expressed as means ± S.E.M, and
analyzed for statistical significance using Student’s t test. P values< 0.05
were considered significant
RESULTS AND DISCUSSION
Antioxidant assay
It is difficult to measure the many different
antioxidants that may be present in plants and hence several techniques have
been established to assess the antioxidant activates of different substances.
Among them, β-carotene bleaching assay, ferric reducing
ability and hydrogen peroxide scavenging assay are commonly used
[18]. We therefore chose these methods to assess the antioxidant activities
of C. procera leaf extracts. In this study, we
determined the ability of C. procera leaf extracts
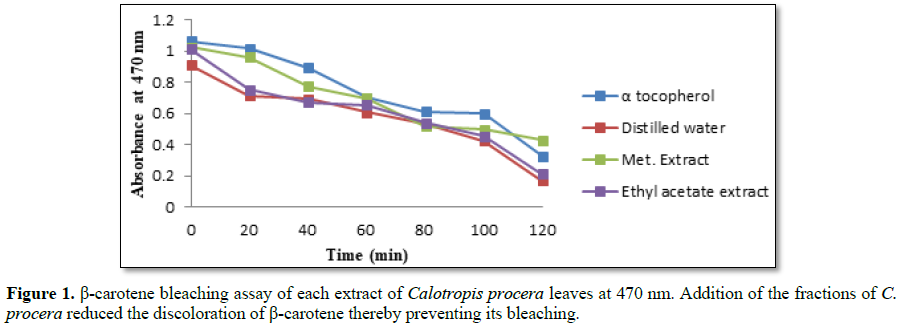
to that of prevent β-carotene bleaching (Figure 1). The methanolic fraction of C. procera showed
comparable inhibition of α-tocopherol on β-carotene bleaching with
concentrations ranging from 50 to 250 μg/mL. The water and ethyl acetate
fractions also inhibited β-carotene bleaching but to a lesser extent. Methanol
is a polar organic solvent and might have extracted both polar and organic
antioxidant molecules in the leaves thereby expressing the highest activity.
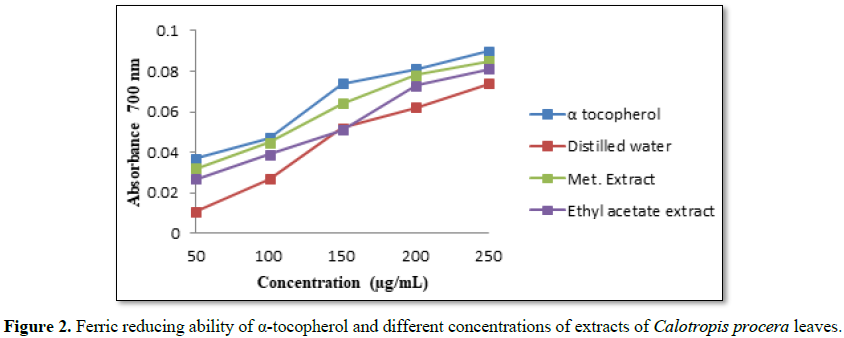
The transformation of Fe3+ into Fe2+ in
the presence of various fractions was measured to determine the reducing power
ability. The reducing ability of a compound generally depends on the presence
of reductones (antioxidants), which exert the antioxidant activity by breaking
the free radical chain by donating a hydrogen atom [19]. In this method,
antioxidant compounds form a colored complex with potassium ferricyanide,
trichloroacetic acid and ferric chloride that was measured at 700 nm. Increase
in absorbance of the reaction mixture indicates an increase in the reducing power
of the sample. There was a concentration-dependent increase in the absorbance
of reaction mixture for all the extracts and standard (Figure 2). Methanolic fraction showed the highest absorbance and
hence the highest reducing power among the fractions. The reducing activities
of the other fractions were in the order α-tocopherol> methanol > ethyl
acetate >water fractions. Our results are in agreement with that
reported by Tadhani et al. [18] which found that methanolic plant extracts had
stronger antioxidant activities than other extracts.
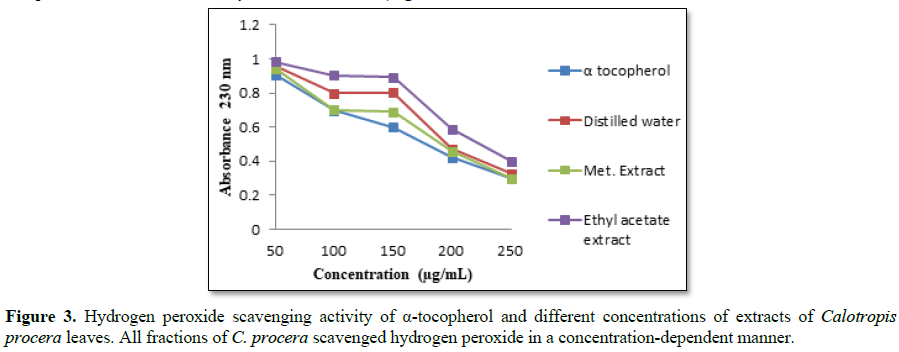
Hydrogen peroxide itself is not particularly reactive
with most biologically important molecules, but is an intracellular precursor
of hydroxyl radicals which is very toxic to cells [20]. Thus, scavenging of H2O2 is
a measure of the antioxidant activity of the fractions. The methanolic extract
had a lower absorbance value among the extracts and hence maximum antioxidant
activity. The antioxidant activity of other extracts was in the order
α-tocopherol> methanol > water > ethyl acetate extracts (Figure 3). All the fractions of C.
procera scavenged hydrogen peroxide which may be attributed to the
presence of phenolic groups that could donate electrons to hydrogen peroxide,
thereby neutralizing it to form water.
Acute toxicity
Since the methanolic extract showed the maximum
antioxidant activities, we tested its toxicity.
In the first 24 h, no
mortality was observed in rats treated with 0.5 and 1g/kg body weight of the
extract. Rats that received higher doses (2g/kg) became sluggish and did not
respond to external stimuli. No changes were however observed in urinary
frequency, salivation, and diarrhoea. The loss of
external stimuli and sluggish movement may be due to the presence of
alkaloids [21] which might have affected the nervous systems of the
rodents [22]. However the LD50 was
not determined in this study.
Subacute toxicity
The experimental group of
rats was examined for weakness, weight loss and loss of hair on the 29th day.
Rats that received 667 mg/Kg body weight of methanolic extracts were found to be weak, lost weight and also lost
their hair. These observations might have been caused by the presence of plant
toxins in the crude methanolic extracts. Results from liver functional tests
showed that all extracts significantly (P>0.05) increased the levels of
serum ALP, ALT, AST, GGT, and bilirubin relative to the control (Table 1).
An increase in these biochemical parameters gives information on
whether the disorder is hepatitic or cholestatic in origin [23]. In this study,
the raised levels of ALP, GGT as well as bilirubin could indicate biliary
obstruction. Also, in such situations, ALP is usually higher than ALT during
biliary obstruction as seen in this study. Although
serum ALP cannot be used to assess acute liver damage or even cirrhosis, it is
an excellent indicator of space-occupying lesion in liver primarily because of
destruction of biliary canaliculi within the liver. When the liver is damaged,
it is unable to conjugate and excrete bilirubin and therefore the increase in
serum bilirubin can also be attributed to the damage of the liver. Damage to
the structural integrity of the liver is also indicated by increase in the
level of serum aminotransferases (ALT and AST) and GGT as these are cytoplamic
in location and are released into circulation after cellular damage [24].
CONCLUSION
From this study, we have shown that methanolic
extracts of C. procera leaf have strong antioxidant activity,
reducing power ability and H2O2 scavenging activity.
As the various fractions of C. procera exhibited different
reactive oxygen species scavenging activities, there may be different
percentages of phytochemical constituents present in the fractions.
Although the LD50 of the extract
could not be determined, the toxic symptoms and the high serum levels of the
biochemical parameters measured indicate that the extracts were toxic. Further
studies to isolate and purify the antioxidant components in the extracts are
warranted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
1.
Beckhauser TF, Francis-Oliveira J, De Pasquale R
(2016) Reactive oxygen species: physiological and physiopathological effects on
synaptic plasticity. J Exp Neurosci 10: 23.
2.
Radak Z, Marton O, Nagy E, Koltai E, Goto S: The
complex role of physical exercise and reactive oxygen species on brain. J
Sports Health Sci 2: 87-93.
3.
Ali S, Ahmed M, Sakandar S, Khan S (2017)
Antioxidants and free radicals in human diseases: current status and future
prospectives. Eur J Pharm Med Res 4: 171-180
4.
Cortez R, Luna‐Vital DA, Margulis D, Mejia E (2017)
Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications.
Compr Rev Food Sci Food Safety 16:180-198.
5.
Salvamani S, Gunasekaran B, Shaharuddin NA, Ahmad
SA, Shukor MY (2014) Antiartherosclerotic effects of plant flavonoids. BioMed
Res Int
6.
Działo M, Mierziak J, Korzun U, Preisner M, Szopa
J, Kulma A (2016) The potential of plant phenolics in prevention and therapy of
skin disorders. Int J Mol Sci 17: 160.
7.
Nieva‐Echevarría B, Manzanos MJ, Goicoechea E,
Guillén MD (2015) 2, 6‐Di‐tert‐butyl‐hydroxytoluene and its metabolites in
foods. Compr Rev Food Sci Food Safety 14: 67-80.
8.
Rahmatullah M, Sultan S, Toma T, Lucky S, Chowdhury
M, et al. (2010) Effect of Cuscutareflexa stem
and Calotropisprocera leaf extracts on glucose tolerance in
glucose-induced hyperglycemic rats and mice. African Journal of
Traditional, Complementary and Alternative Medicines 7: 109-112.
9.
Kumar V, Basu N (1994) Anti-inflammatory activity
of the latex of Calotropisprocera. Journal of Ethnopharmacology 44:123-125.
10.
Setty SR, Quereshi AA, Swamy AV, Patil T, Prakash
T, Prabhu K, Gouda AV (2007) Hepatoprotective activity of Calotropisprocera
flowers against paracetamol-induced hepatic injury in
rats. Fitoterapia 78: 451-454.
11.
Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK
(2002) Evaluation of antioxidant activities and antimutagenicity of turmeric
oil: a byproduct from curcumin production. Zeitschriftfür Naturforschung C
57: 828-835.
12.
Yoon NY, Xie C, Shim K-B, Kim Y-K, Lee DS, Yoon HD
(2011) Antioxidant and Cholinesterase Inhibitory Activities of Antarctic Krill
Eupausiasuperba. Fisheries Aquat Sci 14: 289-293.
13.
Tchimene MK, Nwaehujor CO, Ezenwali M, Okoli CC,
Iwu MM (2016) Free radical scavenging activity of lupeol isolated from the
methanol leaf extract of CratevaadansoniiOliv.(Capparidaceae). Int J
Pharmacogn Phytochem Res 8: 419-426.
14.
Bailey WC, DeRouen TA, Ziskind MM, Greenberg HB
(1975) Autoanalytic (colormetric) determinations of SGOT in isoniazid
recipients are reliable. American Review of Respiratory Disease 111:
237-238.
15.
Matsuzawa T, Katunuma N (1966) Colorimetric assays
for serum alanine transaminase and lactic dehydrogenase using diazonium zinc
salt. Anal Biochem 17:143-153.
16.
Pham NM, Zhenjie W, Morita M, Ohnaka K, Adachi M,
et al. (2011) Combined effects of coffee consumption and serum
γ-glutamyltransferase on serum C-reactive protein in middle-aged and elderly
Japanese men and women. Clin Chem Lab Med 49: 1661-1667.
17.
McComb RB, Bowers GN (1972) Study of optimum buffer
conditions for measuring alkaline phosphatase activity in human
serum. Clin Chem 18: 97-104.
18.
Tadhani M, Patel V, Subhash R (2007) In vitro
antioxidant activities of Stevia rebaudiana leaves and callus. J Food
Compost Anal 20: 323-329.
19.
Kumar RS, Rajkapoor B, Perumal P (2012) Antioxidant
activities of IndigoferacassioidesRottl. Ex. DC. using various in vitro assay
models. Asian Pac J Trop Biomed 2: 256-261.
20.
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free
radicals: properties, sources, targets, and their implication in various
diseases. Indian J Clin Biochem 30: 11-26.
21.
Doshi H, Satodiya H, Thakur MC, Parabia F, Khan A
(2011) Phytochemical screening and biological activity
of Calotropisprocera (Ait). R. Br.(Asclepiadaceae) against selected
bacteria and Anopheles stephansi Larvae. Int J Plant Res 1: 29-33.
22.
Yagiela JA, Dowd FJ, Johnson B, Mariotti A, Neidle
EA (2010) Pharmacology and therapeutics for dentistry-E-Book. (6th edn),
Elsevier.
23.
Hall P, Cash J (2012) What is the real function of
the liver ‘function’tests? Ulster Med J 81:30.
24.
Giannini EG, Testa R, Savarino V (2005) Liver
enzyme alteration: a guide for clinicians. Can Med Assoc J
172: 367-379.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)