764
Views & Citations10
Likes & Shares
It is a commentary on pulmonary pathology of HIV positive patients. An old male patient of 62 years had a history of
HIV. He was treated with grade III dyspnea, cough and hemoptotic sputum. He has
been in antiretroviral treatment for 20 years, with good adherence. But, C-reactive
proteins were seem to be increased when tested in lab. This man’s case is
discussed in this commentary.
A 62 year old male patient was treated for exacerbation of grade III
dyspnea, cough and hemoptotic sputum. He presents as antecedent of hierarchy of
positive serology for HIV (Human Immunodeficiency Virus) since the year 1997,
pneumonia by Pneumocystis jiroveci,
smoking of 6 packets/year, diagnosis of (Chronic obstructive pulmonary disease)
COPD in 2007. He has been in antiretroviral treatment for 20 years, with good
adherence. Increased C-reactive protein is observed in the laboratory. An
obstructive pattern is shown in spirometry. A new chest (computed tomography)
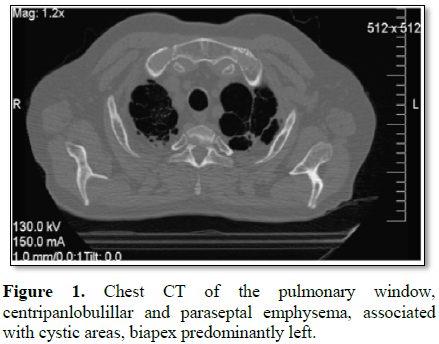
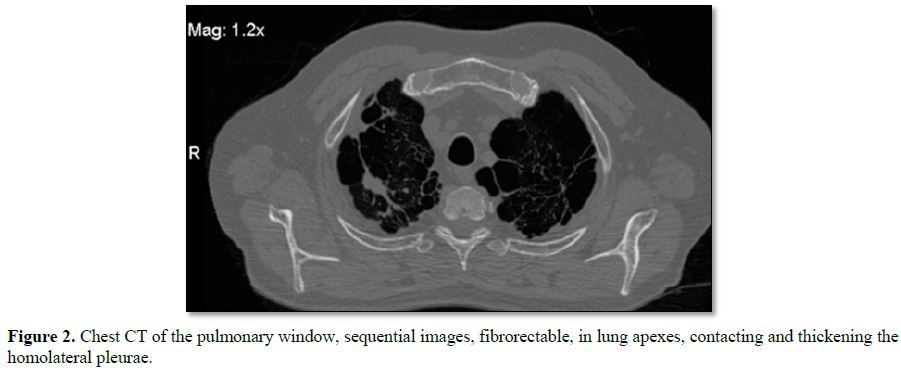
CT scan is requested, which reports in the level of the pulmonary parenchyma
putting in evidence images of secuelar aspect, with fibro-spectral
characteristics at the level of both pulmonary apexes, with no plane of
separation with the homolateral pleura, with which it contacts and thickens,
impressing presenter in its interior small areas of bronchial dilatation.
There are also important signs of centripanlobulillar and paraseptal
enfiesema, with other areas, also with absence of parenchyma corresponding to
pulmonary cysts, being those of higher hierarchy located in the biapical region
and predominantly of the left lung (Figures
1 and 2).
Areas of slight pleural and cisural thickening are also identified,
corresponding to their COPD-based pathology.
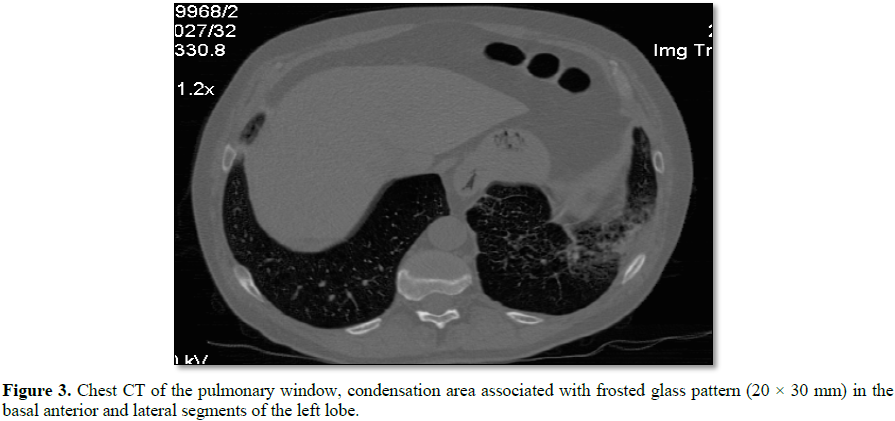
At the level of the anterior and lateral basal segments of the left
lower lobe, a condensation area, associated with a frosted glass pattern, with
linear images is visualized (Figure 3).
The condition is interpreted as an acute exacerbation of its underlying
pathology and is treated with antibiotics, salmeterol puff and fluticasone.
After 20 days a respiratory function test is done. The reduced diffusion
capacity indicates a moderate degree of loss of functional alveolar capillary
surface. It is interpreted as very severe obstructive airway pathology.
The use of tobacco is the main risk factor for COPD and although in the
last years the number of adult smokers decreased, Argentina is still one of the
Latinamerican countries which show an increased use of tobacco.
The relationship between smoking and COPD can be shown with these data:
80% of the COPD patients were smokers and, 1 of 4 smokers has COPD diagnosis.
It is important to consider that not only cigarette can produce COPD,
in fact, other ways of uses of tobacco such as electronic cigarette, shisha and
tobacco heater can lead to COPD too.
Despite the arrival of (antiretroviral therapy) ART,
the epidemic of the human immunodeficiency virus remains a global health crisis
with a high burden of respiratory disease among infected people. While the first complications of the epidemic
were mainly opportunistic infections, improved survival chances showed the
appearance of non-infectious diseases that are associated with chronic
respiratory symptoms and lung impairment.
Obstructive ventilatory defects and reduced diffusion capacity are
common findings in adults, and the association between HIV and chronic
obstructive pulmonary disease is increasingly recognized. People infected with
HIV seem to have an increased risk of obstructive lung diseases, although
whether this represents increased emphysema, chronic bronchitis, asthma or a
combination of these disorders has not been fully evaluated.
Although some of the increase in obstructive pulmonary disease,
especially COPD, may be related to smoking and drug abuse, the apparent risk of
COPD remains high in people infected with HIV. Recent studies of lung
functioning in people infected with HIV have elucidated some factors that may
be important in the pathogenesis of obstructive pulmonary disease in HIV such
as: poor control of HIV contributes to COPD and decreased lung functioning,
metabolic disease and inflammation associated with asthma and airway hyper
reactivity.
Chronic lung disease will become the third most common cause of death
by 2030 in the general population. Early detection and proper management is a
priority to improve the prognosis and patient’s life quality. In turn, the
diagnosis of COPD reinforces smoking cessation, involves a thorough study of
pulmonary function by spirometry, 6-minute walk test (TC6M) and carbon dioxide
diffusion test (CDDT) and screening for lung cancer.
1. Risso K, Guillouet-de-Salvador F, Valerio L,
Puglièse P, Naqvi A, et al. (2017) COPD in HIV-infected patients: CD4 cell
count highly correlated. PLoS One 12.
2. Triplette M, Attia E, Akgün K, Campo M,
Rodriguez-Barradas M, et al. (2017) The differential impact of emphysema on
respiratory symptoms and 6-minute walk distance in HIV infection. J Acquir
Immune Defic Syndr 74: 23-29.
3. Ronit A, Lundgren J, Afzal S, Benfield T,
Roen A, et al. (2018) Airflow limitation in people living with HIV and matched
uninfected controls. Thorax 73: 431-438.
4. Fitzpatrick ME, Kunisaki KM, Morris A (2018)
Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy.
AIDS 32: 277-292.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)