668
Views & Citations10
Likes & Shares
Postpartum depression (PPD) is moderate to severe depression in a woman after she has given birth. It may occur soon after delivery or up to a year later. The time after childbirth, which is supposed to be one of the happiest periods of life, turns to be a sad, painful, and even dramatically so, in the women's life. Moreover, it potentially harms not only the depressive women but also the newborn child [1] and the entire family [2,3]. Sometimes the damage is irreversible [4-7].
There are a lot of unknowns regarding postpartum depression. In spite the fact that the number of studies in the field of PPD is growing in the last decade, the etiology and pathogenesis of the disease are still unclear.
The existing data revealed different psychosocial and physiological correlates. The former includes a history of depression or anxiety during pregnancy, stressful life events or changes during pregnancy, inadequate social support, a history of psychiatric disorders, possible nicotine use [7]. The latter include decreased noradrenaline or serotonin blood level [8] and it's decreased activity in the brain [9], decreased Omega-3 fatty acids [10,11] or 25(OH) Vitamin D [12], fluctuating oxytocin [13] and IL-1beta levels [14,15], abnormalities in the hypothalamic-pituitary-adrenal axis (HPA) [16-20] and others for the PPD development.
A special attention is paid to the role of genetic factors in the etiology of postpartum depression. Several studies have attempted to characterize the specific expressional modifications in patients with mood disorders in general [21-23] and PPD in particular [15,20] and to develop biomarkers for this depressive state. Spijker et al. [21] identified a set of genes whose expressional pattern is strongly correlated with major depressive disorder (MDD). Another study demonstrates altered expression of genes related to inflammatory, apoptotic, and oxidative stress in post-mortem human brain tissue samples (BR 10 area) in MDD [23].
During decade, the involvement the epigenetic factors in the development of perinatal depression has been evaluated [24,25]. For example, Champagne FA in his review provides evidence that epigenetic mechanisms are capable of mediating the inheritance of specific traits across generation including psychiatric disorders such as depression [24]. Guintivano et al. [25] put in data that the DNA methylation associated with PPD risk correlated significantly with estradiol treatment-induced DNA methylation change in blood obtained during the antenatal period of pregnant mood disorder patients [25].
In our recent study, we focused on the possible genetic correlates and causes of PPD [26]. We investigated gene expression in the euthymic women with a history of postpartum depression without any clinical signs of the disease at the onset of the study. We hypothesized that stable modifications in gene expression might be involved in PPD development. That is why it was important to see the expression profile at a “calm time” and not during the stressful time of labor and delivery which can lead to dramatic but temporary changes in genes expression.
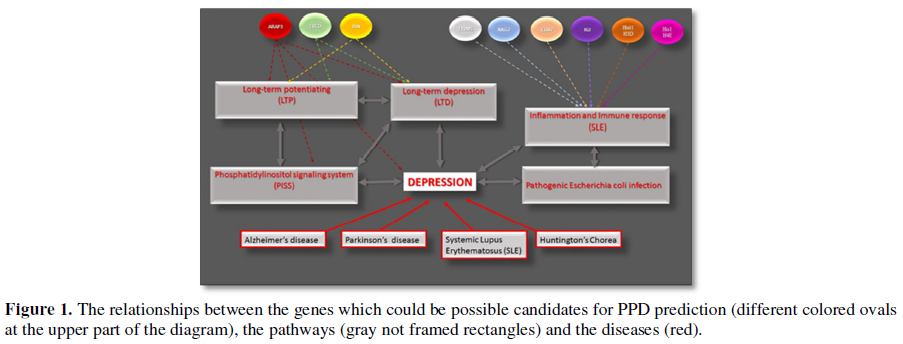
Afterward, the 39 most prominent genes (with a maximal fold of difference in their expression, minimal p-value and possible physiological relationship to the depressive status) were submitted to the Real-Time PCR assay and additional statistical analysis. As a result, we identified nine genes whose alteration may be considered as potential markers for the postpartum depression. These genes are represented in Table 1. The results of the Real-Time PCR analysis support the suggestion that the pathways mentioned above could be involved in the PPD pathogenesis. For example, ARAP3 gene encodes a phosphoinositide binding protein and is critical for the phosphatidylinositol signaling system (PISS) [28,29]. The PISS linked either directly to the mood and neuropathic disorders or through its role in neural plasticity [30]. RIN1 gene is responsible for the RAS effector protein and may serve as an inhibitory modulator of neuronal plasticity, which when altered has been associated with mood disorders, as mentioned above [31,32]. Mutation in TBCD gene is associated with progressive encephalopathy and brain atrophy [33]. The six other genes from our list: NKG7, CD97, IGJ Hist1H3D, Hist1H4e and TRIM5 play an essential role the immune and inflammatory response [34-38]. For example, Hist1H3D and Hist1H4e were shown as important genes of the Systemic lupus erythematosus pathway; and TRIM5 innate immune signaling and this activity is amplified by retroviral infection and interaction with the capsid lattice [37]. The relationships between the described genes, pathways and diseases are summarized in the diagram below (Figure 1).
The importance of these findings is obvious. First, understanding of the molecular events involved in the diseased is crucial for its successful treatment. Second, the aforementioned genes appear to be potential markers of predisposition to depression. Since the prediction of depression at the early stages of pregnancy and appropriate support of the women can prevent the development of the disease, it is critical not to underestimate the importance of the described above findings.
Another important question: are these expressional alterations specific for postpartum depression or do they occurring also in other types of depression. Answers to these questions await further research.
1. Sharma V, Doobay M, Baczynski C (2017) Bipolar postpartum depression: An update and recommendations. J Affect Disord 219: 105-111.
2. O'Hara MW, Wisner KL (2014) Perinatal mental illness: Definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol 28: 3.
3. Malus A, Szyluk J, Galinska-Skok B, Konarzewska B (2016) Incidence of postpartum depression and couple relationship quality. Psychiatr Pol 50: 1135-1146.
4. Farias-Antunez S, Xavier MO, Santos IS (2018) Effect of maternal postpartum depression on offspring's growth. J Affect Disord 228: 143-152.
5. Friedman SH, Resnick PJ (2009) Postpartum depression: An update. Womens Health (Lond) 5: 287-295.
6. Stuart-Parrigon K, Stuart S (2014) Perinatal depression: An update and overview. Curr Psychiatry Rep 16: 468.
7. Beck CT (2001) Predictors of postpartum depression: An update. Nurs Res 50: 275-285.
8. Yildiz G, Senturk MB, Yildiz P, Cakmak Y, Budak MS, et al. (2017) Serum serotonin, leptin and adiponectin changes in women with postpartum depression: Controlled study. Arch Gynecol Obstet 295: 853-858.
9. Avraham Y, Hants Y, Vorobeiv L, Staum M, Abu Ahmad W, et al. (2017) Brain neurotransmitters in an animal model with postpartum depressive-like behavior. Behav Brain Res 326: 307-321.
10. Borja-Hart NL, Marino (2010) Role of omega-3 fatty acids for prevention or treatment of perinatal depression. Pharmacotherapy 30: 210-216.
11. Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, et al. (2014) Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS One 9: e96905.
12. Gur EB, Gokduman A, Turan GA, et al (2011) Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol 79:110-116.
13. Cardaillac C, Rua C, Simon EG, Tatar S, Hepyilmaz I, et al. (2016) Oxytocin and postpartum depression. J Gynecol Obstet Biol Reprod (Paris) 45: 786-795.
14. Shelton MM, Schminkey DL, Groer MW (2015) Relationships among prenatal depression, plasma cortisol and inflammatory cytokines. Biol Res Nurs 17: 295-302.
15. Corwin EJ, Bozoky I, Pugh LC, Johnston N (2003) Interleukin-1beta elevation during the postpartum period. Ann Behav Med 25: 41-47.
16. Kammerer M, Taylor A, Glover V (2006) The HPA axis and perinatal depression: A hypothesis. Arch Womens Ment Health 9: 187-196.
17. Meltzer-Brody S (2011) New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci 13: 89-100.
18. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, et al. (2015) Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 7: 924-930.
19. Bloch M, Daly RC, Rubinow DR (2003) Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 44: 234-246.
20. Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, et al (2005) Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab 90: 695-699.
21. Spijker S, Van Zanten JS, De Jong S, Penninx BW, van Dyck R, et al. (2010) Stimulated gene expression profiles as a blood marker of major depressive disorder. Biol Psychiatry 68: 179-186.
22. Goltser-Dubner T, Galili-Weisstub E, Segman RH (2010) Genetics of unipolar major depressive disorder. Isr J Psychiatry Relat Sci 47:72-82.
23. Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, et al (2011) Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16: 751-762.
24. Champagne FA (2008) Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrin Front Neuroendocrinol 29: 386-397.
25. Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA (2014) Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry 19:560-567.
26. Landsman A, Aidelman R, Smith Y (2017) Overview of microarray technology. Genomics 109: 1-8.
27. Li PC (2011) Overview of microarray technology. Methods Mol Biol 368:3-4.
28. Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, et al. (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9: 95-108.
29. Raaijmakers JH, Deneubourg L, Rehmann H, de Koning J, et al (2007) The PI3K effector Arap3 interacts with the PI(3,4,5)P3 phosphatase SHIP2 in a SAM domain-dependent manner. Cell Signal 19: 1249-1257.
30. Hou L, Klann E (2004) Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24: 6352-6361.
31. Duman RS, Malberg J, Nakagawa S, D'Sa C (2000) Neuronal plasticity and survival in mood disorders. Biol Psychiatry 48: 732-739.
32. Duman RS (2002) Pathophysiology of depression: The concept of synaptic plasticity. Eur Psychiatry 3: 306-310.
33. Miyake N, Fukai R, Ohba C, Chihara T, Miura M, et al. (2016) Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am J Hum Genet 99: 950-961.
34. Becker S, Wandel E, Wobus M, Schneider R, Amasheh S, et al (2010) Overexpression of CD97 in intestinal epithelial cells of transgenic mice attenuates colitis by strengthening adherens junctions. PLoS One 5: e8507.
35. Dorr CR, Oetting WS, Jacobson PA, Israni AK (2018) Genetics of acute rejection after kidney transplantation. Transpl Int 31: 263-277.
36. Kop EN, Adriaansen J, Smeets TJ, Vervoordeldonk MJ, van Lier RAW, et al (2006) CD97 neutralisation increases resistance to collagen-induced arthritis in mice. Arthritis Res Ther 8: R155.
37. Pertel T, Hausmann S, Morger D, Züger S, Guerra J, et al (2011) TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472: 361-365.
38. Turman MA, Yabe T, McSherry C, Bach FH, Houchins JP (1993) Characterization of a novel gene (NKG7) on human chromosome 19 that is expressed in natural killer cells and T cells. Hum Immunol 36: 34-40.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)