2935
Views & Citations1935
Likes & Shares
The need to image and measure the mechanical
properties of skin, scar, tumors, extracellular matrices (ECMs) and wound
tissue has been a goal of researchers since the 1970s. A variety of methods
have been used to image and evaluate the mechanical properties of skin over the
last 40 years including uniaxial and biaxial tensile testing, indentation and
rotational tests, ultrasound elastography (UE), optical coherence tomography
(OCT), optical coherence elastography (OCE), and vibrational analysis combined
with OCT.
We recently reported the use of OCT and
vibrational analysis to identify the differences between skin and scar tissue.
This paper describes the use of vibrational OCT to image and determine the
physical characteristics of the margins of a skin lesion. We report that
differences in the collagen orientation between skin and scar tissue appear to
increase the modulus of the collagen network in scar tissue by a factor of
about 2. Using images generated by OCT, and maps of the modulus as a function
of position, it is possible to identify the edges of scar tissue and other skin
lesions using vibrational OCT.
Keywords: Collagen, Imaging vibrational optical coherence
tomography (VOCT), Optical coherence tomography (OCT), Mechanical properties,
Decellularized dermis, Skin, Scar, Scar margins, Modulus, Natural frequency,
Imaging vibrational optical cohesion tomography Imaging, Vibrational optical
coherence tomography (VOCT),
INTRODUCTION
Mechanobiology and
physiology play important roles in normal tissue homeostasis as well as in
repair, regeneration and disease processes [1-5]. Mechanical forces in
extracellular matrix (ECM) not only influence the storage and dissipation of
mechanical energy during locomotion and blood flow [6-9], they also influence
gene expression and tissue remodeling [10]. Extracellular matrices (ECMs) found
in musculoskeletal, cardiovascular, dermal and other tissues are under tension
during normal physiologic loading, even in the absence of external forces
[1,2]. This tension not only fulfills cosmetic functions, but also creates a
state of dynamic loading at the collagen fibril-cell interface and at cell-cell
attachment points that stimulate conversion of mechanical work (a force moving
an object through a distance) into chemical energy (synthesis of high molecular
weight cellular components) [1-3].
Changes in the mechanical
properties of ECM are known to accompany the onset and progression of several
highly prevalent diseases, including atherosclerosis, cirrhosis, and cancer. At
least one publication underscores the relationship between cancer and tissue
fibrosis [11]. A technique to image and
measure of the mechanical properties of tissues may enable early diagnosis of
some of these conditions.
Extracellular matrix in the
tumor stroma is characterized by remodeling and stiffening; tissue stiffness
has been used to detect cancer [12,13]. ECM stiffening has been reported to
enhance cell growth and survival, and promote cell migration [14]; ECM rigidity
disrupts tissue morphogenesis by increasing cell tension [15]. Reduction of
cell tension has been reported to repress the malignant behavior of mammary
epithelial cells (MECs) and normalized the behavior of breast cancer cells in culture
[15]. ECM stiffening in tumors may be related to changes in the phosphorelay
pathways, and ECM tension has been postulated to drive tumor formation [11]. The need to measure the mechanical properties of skin, scar, tumors,
extracellular matrices (ECMs) and wound tissue has been a goal of researchers
since the 1970s. The pioneering work of Yamada [20] and Fung [21] illustrated
how difficult this goal would be since the behavior of human ECM depends on
strain-rate, direction of testing and is time-dependent [3]. A variety of methods
have been used to evaluate the mechanical properties of skin over the last 40
years including uniaxial and biaxial tensile testing, indentation and
rotational tests, ultrasound elastography (UE), optical coherence tomography
(OCT), optical coherence elastography (OCE), and vibrational analysis combined
with OCT [22-25]. Many of these techniques require the assumptions that the
material is linearly elastic, Poisson’s ratio is close to 0.5 and that
viscoelasticity does not dramatically affect the resulting properties of the
tissue. However, skin is a non-linear material that is viscoelastic and has
upward curvature to the stress-strain curve. This fact makes determination of
the stiffness (tangent to the stress-strain curve) and other mechanical
properties very difficult to quantitatively analyze since the tangent to the
stress-strain curve is constantly changing [22,24,25]. However, despite all of
these problems, there is a need to be able to characterize the mechanical
properties of skin, since this would give clinicians valuable information about
pathological changes that occur during disease processes, the stage of diabetic
skin ulcers and the efficacy of cosmetic treatments. In this paper, we will
discuss the results of a study to image using OCT and determine the mechanical
properties of scar tissue and surrounding skin using vibrational analysis. This
paper describes the use of vibrational OCT to image and to determine the
physical characteristics of the margins of a skin lesion.
METHODS
Calibration Sample
Preparation
Human decellularized dermis
was obtained from allograft tissue as described previously [24-26].
Decellularized human dermal samples were tested after immersion in phosphate
buffer solution as described elsewhere [24-26]. All samples were tested wet
after soaking in phosphate buffer solution at pH 7.4 for at least 30
minutes. Processing and testing steps
were conducted at 22oC.
Human skin and normal scar
tissue were evaluated in vivo to
demonstrate the clinical use of the OCT and vibrational techniques. The scar tissue was the result of a
small thermal burn wound smaller than the size of a dime that was depigmented
after healing (Figure 1). Mechanical Testing
Incremental
Stress-Strain Tensile Measurements in
Vitro
Samples were tested in
uniaxial tension at 22oC by adding a strain increment and then
measuring the load before an additional strain step was added as described
previously [23-26]. Varying axial deformations of between 1 and 14% were
applied through adjustment of a graduated translation stage. The resulting axial force (F) was measured by the force gage and
recorded for subsequent calculations.
Stress values were calculated from the force divided by the
cross-sectional area. Strains were calculated by dividing the change in length
by the original length based on the movement of the translational stage after
each strain increment was added. The tensile modulus was calculated from a tangent
drawn to the stress-strain curve at the strain increment used.
OCT and Vibrational Analysis in Vitro
Transverse forces were
applied to the sample by positioning an acoustic loudspeaker (Intervox
S225RA-40) beneath the sample. A
function generator (Agilent) was used to drive the speaker with sinusoidal
waveforms at varying amplitude and frequency.
Transverse sample
displacement was measured by spectral-domain optical coherence tomography
(SD-OCT), a non-contact, interferometric technique as discussed previously
[25,26]. The SD-OCT system uses a fiber-coupled superluminescent diode light
source with 1325 nm center wavelength and 100 nm bandwidth (full-width at
half maximum) [25,26].
The resonant frequency of
each sample was initially estimated at a signal point by measuring the
transverse displacement resulting from sinusoidal driving frequencies ranging
from 50 Hz to 1000 Hz, in steps of 50 Hz. Once the region where the maximum
frequency was identified, smaller steps of 10 Hz were used to more accurately
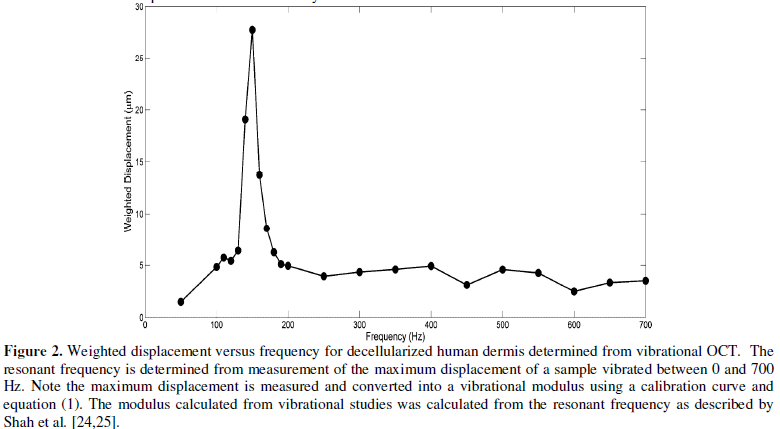
identify the peak frequency and the actual resonant frequency, fn (Figure 2).
![]() (1)
(1)
The modulus from in vitro vibrational studies was
determined using equation (1) where m,
L and A are the sample mass, length and cross-sectional area.
In Vivo
Determination of the Resonant Frequency of Skin and Scar Tissue
In vivo studies on the mechanical
properties of skin and healed scar tissue were conducted by hard wiring a 24 mm
x 14 mm rectangular speaker (Digi-Key, Thief River Falls, MN) to a Samsung cell
phone. A frequency generating app was downloaded from the Google Play Store
onto the cell phone. This app was capable of driving the speaker between 10 and
20,000 Hz. The speaker was taped to the skin using surgical tape and it was
used to generate a sinusoidal sound wave that vibrated the skin. During in vivo measurements, no sensation of
the light or sound impinging on the skin was felt. The sound intensity was low
enough so that it could not be detected unless the speaker was placed near the
subject’s ear to make sure it was energized.
The Digi-Key speaker was
used for in vivo measurements in
place of the Intervox speaker described for the in vitro studies above. The speaker was located about 2.5 cm from
the where the incident light beam contacted the skin and did not interfere with
impingement of the light on the skin. The location of the incident beam on the
skin influenced the extent of the displacement but not the resonant frequency
(data not shown). The optical signal
generated by vibrating the skin with the Digi-Key speaker was then processed in
the same manner as was done for in vitro
studies and the resonant frequency was obtained by determination of the
frequency at which the displacement was maximized. Measurements made with the
Digi-Key speaker were made on decellularized human dermis in vitro and the resonant frequency determined using this speaker
was similar to that measured with the Intervox speaker.
Calibration Studies
A variety of samples made
from silicone rubber, decellularized dermis, and chemically modified
decellularized dermis were tested in uniaxial tension and using vibrational
analysis to establish a calibration curve between the moduli calculated from
tensile measurements and those derived from vibrational measurements in vitro. These results have been
published elsewhere [25,26].
The relationship between the
modulus measured using vibrational and tensile measurements was reported to be
approximately linear and the equation of the line was found to be:
Ev=1.026
Et + 0.0046 (2)
where, Ev and Et are the
moduli measured using vibrational and tensile measurements, respectively and
are in MPas. The correlation coefficient between these moduli is 0.984 as previously
reported [25,26]. The relationship between tensile and vibrational moduli was
approximated using equation (2). The material behavior was reported to be
reversible for strains less than about 14% for up to three cycles of tensile
testing [25,26].
Imaging
Photographic and OCT images
of skin and scar were made using a Samsung cell phone, and a Lumedica OQ labscope1.0
(Lumedica, Inc., Durham, NC) operating in the scanning mode, respectively.
RESULTS
Vibrational OCT was
used to measure the mechanical properties of human skin and scar in vivo. The results of previously
published data on decellularized dermis and silicone rubber were used to
calibrate the in vitro and in vivo studies. The resonant frequency
was measured from vibrational OCT studies as shown in Figure 2. Resonant
frequencies were obtained by determining the frequency at which the maximum
displacement was observed by measurements at a single point (Figure 2).
For decellularized dermis, the value of the resonant frequency at 5% strain was
calculated to be 150 Hz as reported previously [25,26]. The modulus of
decellularized dermis at 5% strain was calculated from equation (1) as
previously described and compared to the tensile modulus using equation (2) and
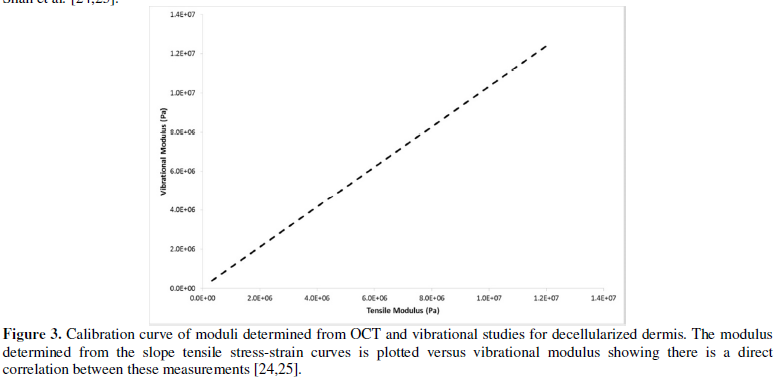
the calibration curve (Figure 3) as described previously [25,26].
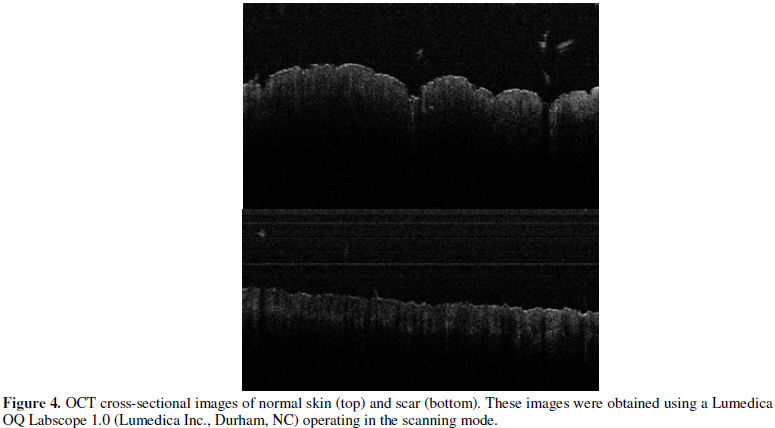
Photographic and OCT images
of both skin and scar are shown in Figures 1 and 4, respectively.
Photographic images showed that the scar is approximately 7 mm in diameter and
was clearly demarcated from the surrounding skin by differences in
pigmentation. The edges of the scar were marked N, S, E and W to determine
whether differences in the mechanical properties could be measured in
comparison to the normal skin (skin) and the scar tissue proper (scar). OCT
scanning images of the skin and scar cross-sections are shown in Figure 4.
The normal skin appeared to have surface hills and valleys while the scar
tissue appeared smoother in texture of the surface.
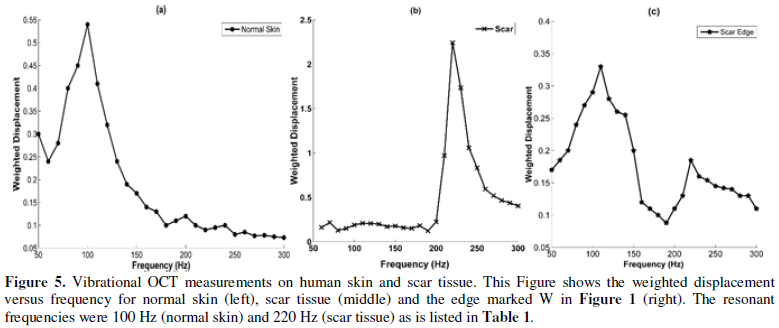
Plots of weighted
displacement versus frequency for normal skin, scar, and one of the edges of
the scar are shown in Figure 5. The plot of weighted displacement versus
frequency for scar tissue is shifted to the right as compared to that of normal
skin (higher resonant frequency). The resonant frequency of normal skin (Skin)
was found to be 100 Hz while that of scar tissue (Scar) was 220 Hz. The edges
of the scar (N, S, E, W) had resonant frequencies of both normal skin (90-100
Hz) and scar tissue (220-230 Hz) as shown in Figure 5 and in Table 1.
The resonant frequency for
decellularized dermis was found to be dependent on sample thickness. However,
the product of E, the modulus, and the sample thickness, d, was found to be
related to the resonant frequency as shown in Figure 6. The resonant
frequency and thickness in vivo were
determined from vibrational OCT and the modulus was calculated from Figure 6.
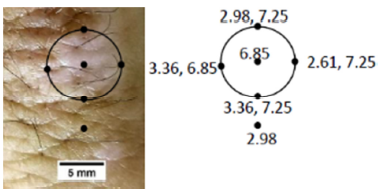
The calculated modulus of normal skin was determined to be 2.98MPa and that of
scar tissue was 6.85 MPa. These values
are close to the values reported for decellularized dermis at 5% strain (skin)
and 14% strain (scar) reported previously [25,26]. The edges of the scar had
moduli that were similar to both normal skin and scar tissue.
DISCUSSION
The inability of
researchers and clinicians to understand how to correlate changes in images of
skin lesions with the content and organization of the ECM hampers the
development of new non-invasive methods to understand and diagnose the
pathogenesis and nature of skin disorders. While the mechanical properties of
skin are complex, much progress has been made in understanding the strain-rate
dependence, non-linearity and compressibility of this tissue [25,26].
We recently reported the use
of OCT and vibrational analysis to identify the differences between skin and
scar tissue [25-27]. The correlation between modulus measurements on
decellularized human dermis made using standard tensile testing in vitro and vibrational OCT suggest
that measurements made using vibrational OCT give results that are consistent
with tensile testing, a “gold standard method” for measuring mechanical
properties of skin. Without comparison to a standard technique, moduli
measurements made with new methods such as vibrational OCT cannot be validated.
Figure 7. Photographic image and modulus values for
normal human skin and scar. Photographic image for skin and scar are shown on
the left. Modulus values for normal skin (2.98 MPa) and scar (6.85 MPa) are
shown on the right as well as for the points N,E, S and W. The edges of the
scar have two resonant frequencies and modulus values corresponding to that of
normal skin and scar were determined from Figure
6 and are listed in Table 1.
Tensile incremental and
constant rate-of-strain measurements made on tissues have been the gold
standard for determination of the mechanical properties of tissues for decades
[20-22]. Many techniques require the assumption that the tissue density is near
1.0 and that Poisson’s ratio is 0.5. The later has been shown to vary between
0.38 and 0.75 for decellularized dermis [25-28] and the tissue density for
human skin is about 1.2 g/cc. The assumption that Poisson’s ratio is 0.5 will
lead to errors in modulus calculations. However, by measuring the resonant
frequency using vibrational analysis and OCT and using equations (1) and (2),
the tensile modulus can be determined for skin without the need to make any
assumptions. In addition, the results of vibrational studies on pig skin
indicate that properties of both the elastic and collagen fiber networks can be
measured in the same experiment, which constitute the major contributors to
mechanical behavior of skin [1,2,29].
By calibrating our in vivo system for measuring the
resonant frequency of normal and scar tissue, we are able to show that the
resonant frequency of scar tissue is 220-230 Hz, an increased value from than
that found for normal skin (90-100 Hz). The resulting increase in modulus for
scar tissue compared to normal skin is consistent with a previous report that
suggests that hypertrophic scar tissue appears stiffer than normal skin due to
its inability to deform during mechanical loading [28]. The fact that the edges
of the scar tissue show resonant frequencies of both normal skin and scar
tissue suggest that two different collagen networks exist at the edges of the
scar tissue. Normal skin contains an almost biaxial network of collagen fibers
that are able to orient under increasing loads with the direction of tension
[1,2]. The modulus of the collagen network in normal skin increases with
increased deformation until the network becomes fully recruited with the
loading direction. In contrast, the modulus of scar tissue is much higher at
low strains since the collagen fibers in scar tissue are more disorganized and
have difficulty reorienting during tensile deformation. In is likely that the
edges of the scar have a combination of normal skin with biaxial orientation
and disorganized collagen in the scar that give rise to both the normal skin
modulus as well as the higher scar modulus (see Figure 7).
The ability to measure
differences in the collagen orientation suggests that vibrational OCT may be
useful in imaging and measuring mechanical differences that occur at the
interface between normal skin and skin lesions. This may be useful in
characterizing the margins of benign and malignant skin lesions as well as the
extent of healing of diabetic skin ulcers and other wounds. The technique may
also be useful in evaluating the efficacy of treatments to skin and tumors.
CONCLUSIONS
Using vibrational OCT the
resonant frequency and moduli of collagen in skin and scar tissue can be
measured non-invasively and non-destructively. The numbers generated reflect to
a first approximation the elastic moduli and do not depend on measurement of
other parameters. Differences in the collagen orientation between skin and scar
appear to alter the modulus of the collagen network by a factor of about 2. The
technique in vitro is calibrated
using incremental tensile measurements and vibrational OCT results on
decellularized human dermis. Using images generated by OCT, and maps of the
modulus as a function of position, it may be possible to determine the margins
of scars, tumors, as well as to evaluate the effects of cosmetic treatments to
the skin.
- Silver FH, Siperko LM, Seehra GP (2002) Mechanobiology of force
transduction in dermis. Skin Res Technol 8: 1-21.
- Silver FH, DeVore D, Siperko LM (2003) Invited Review: Role of
mechanophysiology in aging of ECM: effects of changes in mechanochemical
transduction. J Appl Physiol 95:
2134-2141.
- Silver FH (2006) Mechanosensing and Mechanochemical Transduction in
Extracellular Matrix, Springer, NY.
- Silver FH, Siperko LM (2003) Mechanosensing and Mechanochemical
Transduction. Crit Rev Biomed Eng 31: 255-331.
- Moore SW (2003) Scrambled eggs: mechanical forces as ecological
factors in early development. Evolut Develop 5: 61-66.
- Silver FH, Snowhill PB, Foran D (2003) Mechanical behavior of vessel
wall: A comparative study of aorta, vena cava, and carotid artery. Ann
Biomed Eng 31: 793-803.
- Freeman JW, Silver FH (2004) Elastic energy storage in unmineralized
and mineralized extracellular matrices (ECMs): A comparison between
molecular modeling and experimental measurements. J Theor Biol 229:
371-381.
- Horvath I, Foran DJ, Silver FH (2005) Energy Analysis of Flow
Induced Harmonic Motion in Blood Vessel Walls, Cardiovasc Eng 5: 21-28.
- Freeman JW, Silver FH (2004) Elastic energy storage in unmineralized
and mineralized extracellular matrices (ECMs): A comparison between
molecular modeling and experimental measurements. J Theor Biol 229:
371-381.
- Chiquet M (1999) Regulation of an extracellular matrix gene
expression by mechanical stress. Matrix Biol 18: 417-426.
- Leventhal KR, Yu H, Kass L, Latkins JN, Egeblad M, Erler JT, Fong
SFT, Csiszar K, Giacci A, Weninger W, Yamauchi M, Gassar DL, Weaver, VM
(2009) Matrix crosslinking forces tumor progression by enhancing integrin
signaling. Cell 139: 891-906.
- Butcher DT, Allston T, Weaver VM (2009) A tense situation: forcing
tumor progression. Nat. Rev. Cancer 9: 108-122.
- Sinkus R, Lorenzen J, Shrader D, Lorenzen M, Dargatz M, Holtz D
(2000) High-resolution tensor MR elastography for breast tumour detection.
Phys Med Biol 45: 1649-1664.
- Lo GM, Wang HB, Dembo M, Wang YL (2000) Cell movement is guided by
the rigidity of the substrate. Biophys J 79: 144-152.
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rosenberg GI, et al.
(2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:
241-254.
- Snowhill PB, Foran DJ, Silver, FH (2004) A mechanical model of
porcine vascular tissues-Part I. Determination of macromolecular component
arrangement and volume fractions. Cardiovasc Eng 4: 281-294.
- Snowhill PB, Silver FH (2005) A mechanical model of porcine vascular
tissues-Part II: Stress-strain and mechanical properties of juvenile
porcine blood vessels. Cardiovasc Eng 5: 157-169.
- Jodele S, Blavier L, Yoon JM, Declerck, YA (2006) Modifying the soil
to affect the seed: role of stromal-derived matrix metaloproteinases in cancer
progression. Cancer Metastasis Rev 25: 35-43.
- Ramaswamy, S, Ross, KN, Lander, ES, and Golub, TR (2003) A molecular
signature of metastasis in primary solid tumors. Nat Genet 33: 49-54.
- Yamada H (1970) Strength of Biological Materials, Williams and Wilkins,
Baltimore, MD.
- Fung YC (1993) Biomechanics: Mechanical Properties of Living Tissue,
Second Edition, Springer, NY.
- Dunn MG, Silver FH (1983) Viscoelastic behavior of human connective
tissues: Relative contribution of viscous and elastic components. Conn Tis
Res 12: 59-70.
- Silver FH, Shah R (2016) Measurement of mechanical properties of
natural and engineered implants. Adv Tissue Eng Reg Med 1: 1-9.
- Shah R, DeVore D, Pierce MG (2016) Morphomechanics of dermis-A
method for non-destructive testing of collagenous tissues. Skin Res Tech.
- Shah R, Pierce MC, Silver FH (2017) A method for non-destructive
mechanical testing of tissues and implants. J Biomed Mat Res 105A: 5-22.
- Silver FH, Freeman J, DeVore D (2001) Viscoelastic properties of
human skin and processed dermis. Skin Res Tech 7: 18-23.
- Silver FH, Silver (2017) Non-invasive viscoelastic behavior of human
skin and decellularized dermis using vibrational OCT. Derm Clin Res 3:
174-179.
- Dunn MG, Silver FH, Swann DA (1985) Mechanical analysis of
hypertrophic scar tissue: Structural basis for apparent increased
rigidity. J Invest Dermatol 84: 9-13.
- Shah RG, Silver FH (2017) Vibrational analysis of extracellular
matrix scaffolds: comparison of skin, dermis, cartilage and subchondral
bone. Adv Tiss Eng Regen Med 2: 00048.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of AIDS (ISSN: 2644-3023)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Oncology Clinics and Research (ISSN: 2643-055X)