899
Views & Citations10
Likes & Shares
T lymphocytes, an
indispensable component of the adaptive immune system, protect our bodies
against infections, tumors etc. T cell progenitors are produced in the bone
marrow and their sequential differentiation occurs in the thymus. The most
immature cells, CD4-CD8- thymocytes give rise to the CD4+CD8+ cells. These
cells undergo positive and negative selection, after which only the
non-self-reactive and immune competent T cells survive. Subsequently, the phenotypically
and functionally mature CD4+CD8- or CD4-CD8+ T cells egress from the thymus
into the periphery as recent thymic emigrants. For constant efflux of selected
thymocytes with a diverse T cell receptor repertoire into the periphery, stable
functioning of the thymus is necessary. However, the thymus undergoes reduction
in its cellularity, known as thymic atrophy, due to its exceptional sensitivity
to stress and other factors. Thymic atrophy in humans occurs physiologically
with ageing and pregnancy, and during stress conditions, including
malnutrition, infections, cancer chemotherapies etc. Thymic atrophy is also
observed in patients with graft-versus-host disease, Down’s syndrome and sudden
infant death syndrome. Thymic output reduces with age and it is perceived to be
of lesser significance later in life. However, studies have revealed that the thymus
continues to function in older people. In fact, thymic functioning is crucial
in scenarios post transplantation, chemotherapy and antiretroviral therapy.
This review focuses on conditions where the human thymus atrophies and
interventions (e.g. supplementation of thymulin, antioxidants, IL-7, growth
hormone, ablation of androgens, etc.), including clinical trials, which rescue
thymic cellularity and/or enhance thymic output.
Keywords: IL-7, T cell
development, Sex steroids, Thymus, Thymic atrophy, Thymulin
Abbreviations:
aGVHD: Acute Graft-Versus-Host
Disease; AIRE: Autoimmune Regulator; BMT: Bone Marrow
Transplantation; cTECs: Cortical TECs; Cy: Cyclophosphamide; Dll4: Delta-like
4; DN: Double Negative; DP: Double Positive; ETP: Early Thymic Progenitors;
FSP: Fibroblast Specific Protein 1; GC: Glucocorticoid; GH: Growth Hormone;
GVHD: Graft-Versus-Host Disease; HAART: Highly Active Antiretroviral Therapy;
HSCT: Hematopoietic Stem Cell Transplantation; IL-7R: IL-7 Receptor; ISP:
Immature Single Positive; KGF: Keratinocyte Growth Factor; LHRH: Luteinizing
Hormone-Releasing Hormone; mTECs: Medullary TECs; RTE: Recent Thymic Emigrants;
sjTREC: Signal Joint TCR rearrangement Excision Circles; SP: Single Positive;
TCR: T Cell Receptor; TEC: Thymic Epithelial Cells; TRA: Tissue-Restricted
Antigens; TREC: TCR Rearrangement Excision Circles
INTRODUCTION
T cell development,
selection and maturation occur in the thymus, a primary lymphoid organ. The
thymus was named by Galen (129-210 or 216 AD) because of its structural
resemblance to the leaf of the thyme plant [1]. For years it was considered to
be a vestigial organ till it was reported as the site of T cell development,
making it one of the last major immune organs to be discovered [2,3]. Evolution
suggests that it is one of the newer organs to appear, observed for the first
time in fish [4]. The thymus is highly conserved in terms of developmental
origin, anatomical location and function in all jawed vertebrates, i.e.,
gnathostomes [5]. Interestingly, in the jawless fish lampreys, primitive
thymus-like lympho-epithelial structures called thymoids are found. These
structures express the transcription factor, FOXN4,
the ortholog of
forkhead
T cell development
occurs via cell-cell interactions between thymocytes and cells that constitute
the stromal cell network, e.g. thymic epithelial cells (TECs), dendritic cells,
B cells and macrophages. T cell progenitors originating from the bone marrow
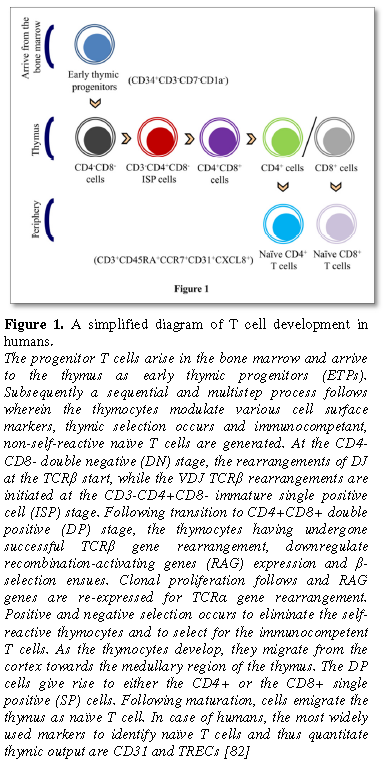
arrive into the thymus as early thymic progenitors (ETPs). The development of T
cells can be monitored using cell surface expression of the T cell
co-receptors, CD4 and CD8, wherein CD4-CD8- (double negative, DN) are the most
immature thymic subset, that give rise to the CD4+CD8+ cells (double positive,
DP) via an intermediate cell population, the immature single positive cells
(ISP) (Figure 1). T cell selection in the thymus is spatially
compartmentalized and is executed primarily by the TECs, wherein the cortical
TECs (cTECs) and the medullary TECs (mTECs) mediate positive and negative
selection respectively. During positive selection, the DP thymocytes that
express the T cell receptor (TCR) are capable of recognizing self-peptides on
cTECs receive survival signals and develop into CD4+/CD8+ single positive (SP)
thymocytes. More than 90% of thymocytes do not get positively selected and
undergo apoptosis. The majority of negative selection occurs on SP thymocytes
and is mediated by mTECs which express a wide variety of tissue-restricted
antigens (TRA). The developing thymocytes that are reactive to self-antigen-MHC
complexes are eliminated, thus preventing the emergence of autoimmune T cells.
The expression of TRA is regulated by a transcription regulator, the autoimmune
regulator (AIRE), which is expressed in ~30% of mTEC and a subset of B cells.
AIRE-deficient mice display decreased TRA expression and autoimmunity including
autoantibody production and autoimmune T cells in the periphery [8,9].
Following negative selection, the SP cells mature and the cells egress from the
thymus as phenotypically and functionally competent naïve cells, knows as recent
thymic emigrants (RTEs) (Figure 1). The fitness or the output of the
thymus is determined by the number of RTEs in the periphery. Quantification of
signal joint TCR rearrangement excision circles (sjTREC) is one of the most
commonly used methods of measuring RTEs. sjTRECs are circular extra-chromosomal
by-products generated during the TCRα chain rearrangement. These DNA circles do
not replicate and thus their frequency reduces with every cell division [10].
Apart from sjTRECs, in humans CD31 (PECAM-1), a member of the Ig superfamily,
is used to quantify RTEs. Human RTEs are CD31+, contain sjTRECs and express
CD45RA. Peripheral expansion of RTEs give rise to central naïve T cells that
lack sjTRECs and only express CD45RA but not CD31 [11]. Contrary to popular
belief, sjTREC studies in humans have demonstrated that the thymus is active
throughout life, with the output of the thymus declining abruptly only in the
10th decade [12].
The thymus is one
of the most sensitive organs to atrophy, i.e., loss in thymic cellularity and
its architecture and output. It is widely perceived that the activity of the
thymus is known to reduce starkly post puberty and as a consequence is not
beneficial further in life. Although the peripheral naïve T cell pool in humans
is maintained almost independent of the thymus post adolescence [13], the
thymus continues to remain active, and its functioning is vital for T cell reconstitution
post highly active antiretroviral therapy (HAART) in HIV-infected patients,
bone marrow transplantation (BMT) and chemotherapy [14]. Recently an
immunological model has proposed that there is a strong correlation between
incidences of infectious diseases and cancer and T cell output, making thymic
atrophy a significant risk factor [15]. This review aims to discuss the
conditions in which the human thymus atrophies followed by therapeutics to
rejuvenate it. We have also cited the most recent developments in the field
using animal models wherever necessary.
THYMIC ATROPHY IN HUMANS
Thymic atrophy
often results in reduced thymic output and naïve T cell numbers in the
periphery along with a restricted TCR repertoire. These may result in dampened
responses to novel pathogens, reduced T cell reconstitution post
transplantation, poor response post vaccine challenge and decreased tumor
surveillance [16]. Thymic atrophy is well known to physiologically occur during
ageing and pregnancy and also during myriad clinical conditions. Many factors,
listed elsewhere [14], can either independently or in concert cause thymic
atrophy.
Some of the
instances where the human thymus undergoes atrophy or its output reduces are as
follows: Cortisol, secreted by the adrenal cortex, is the primary
glucocorticoid (GC) produced by the body. GCs induce thymocyte apoptosis in a
calcium-, ATP- and caspase-dependent manner [17]. Astronauts returning from
space flights had elevated amounts of cortisol in urine and plasma and reduced
thymic output as measured by TREC content [18]. Thymic damage can also result
in cases including acute graft-versus-host disease (aGVHD). Allogeneic
hematopoietic stem cell transplant (HSCT) can lead to aGVHD, which increases
the risks of chronic GVHD, a major factor causing morbidity and mortality in
BMT recipients [19]. aGVHD reduces the cellularity of the intrathymic AIRE+mTEChigh
cells, resulting in emergence of autoimmunity [20]. Severe reduction in thymic
output as measured by sjTREC content is also observed in patients of Down’s
syndrome, which is caused due to an autosomal disorder [21]. Thymus
transcriptome studies have revealed that the reduced thymic output is due to
the hypoexpression of genes related to antigen processing and presentation, T
cell differentiation and selection and AIRE-partner genes and not due to
premature ageing as previously perceived [22]. Another example of thymic
atrophy is sudden infant death syndrome (SIDS), which is the leading cause of
infant death within the first year of life. Exogenous stressors are
hypothesized to contribute towards the phenomenon and the health of the thymus
was considered as a parameter in a study. Thymic from SIDS infants displayed
reduced proliferation of thymocytes and enhanced macrophage activity, hallmarks
observed during stress-induced inflammation [23].
THERAPEUTICS KNOWN TO REJUVENATE THE THYMUS
IN ADULTS
Thymulin
Thymulin, the
thymic peptide hormone, is secreted by TECs [24]. Zinc acts as thymulin’s
cofactor, making its presence indispensable for the peptide hormone’s activity [25].
In malnourished children, zinc supplementation augments their thymus size [26],
possibly by increasing the levels of active thymulin. With ageing, the zinc
pool progressively depletes in humans, which may contribute towards
age-associated thymic atrophy due to reduced activity of thymulin as
demonstrated in old mice [27]. Extra thymic production of thymulin is reported
in macrophages and fibroblasts under stress conditions including heat, oxidative
stress, apoptosis and necrosis [28].
Acute zinc
deficiency is found in patients infected with HIV at different stages of the
disease [29]. In fact, the zinc-bound active form of thymulin is very low or
undetectable in HIV-positive pediatric patients who progress to AIDS [30]. Zinc
deficiency and low CD4+ T cell counts are significant risk factors towards
incidence of opportunistic infections in HIV-infected patients. Accordingly,
monitored supplementation of zinc in the diet of late stage HIV-positive patients
along with HAART leads to complete reduction of infections by Candida aesophagea, Pneumocystis carinii, etc. [31]. Long-term zinc supplementation in
the diet delays immunological failure and reduces diarrhea by more than half in
HIV-infected adults [32]. In addition, keeping the above mentioned studies into
perspective, the widely reported thymopoietic properties of growth hormone
[33,34] may be due to its ability to increase thymulin secretion [35].
IL-7
IL-7 is a
non-hematopoietic cell-derived, non-redundant lymphopoietic cytokine. Its roles
in T cell development are evolutionarily conserved, from lower vertebrates to
humans. Mutations in the IL-7 signaling pathway acutely affect thymopoiesis in
zebrafish [36]. A homeostatic mechanism exists to keep the IL-7-expressing
cells in check. After positive selection into the CD4 lineage, the frequency of
IL-7-expressing TECs reduces moderately, whereas negative selection results in
a prominent loss of these cells [37]. IL-7 is required during various stages of
T cell development, maturation and survival and its multifaceted functions are
summarized in Table 1.
Defective IL-7 receptor (IL-7R) signaling is observed
in patients suffering from severe combined immunodeficiency [38,39].
Strikingly, IL-7R deficiency in mice causes absence of both B and T cells,
while in humans the B cells are present [38,39]. Continuous thymic activity is
required for IL-7-mediated proliferation of naïve CD4+ T cells. Dampened
IL-7-driven homeostatic proliferation is observed in CD31+ naïve CD4+ T cells
from individuals thymectomized in early childhood during corrective cardiac
surgery [40]. There are numerous factors which contribute to IL-7-mediated
thymopoiesis (Table 1).
IL-7 is expressed by TECs, which supports survival
and maturation of thymocytes [41]. Studies in mice have demonstrated that the
frequency of cells expressing high amounts of IL-7 reduces with age, probably
contributing to age-associated thymic atrophy [42]. Administration of IL-7
directly increases TREC content in adult as well as in fetal thymus, possibly
due to increased TCR rearrangement [43], although contradictory observations
have also been reported in ageing mice [44]. Interestingly a correlation of
physical activity to thymic output has been recently reported. Older adults who
maintained a high level of physical activity (cycling) had comparable levels of
naïve T cells and RTE to that of young adults. Their age-matched less
physically-active counterparts had lower serum levels of thymopoietic hormones,
IL-7 and higher levels of IL-6, which is known to induce thymic atrophy [45].
Lower mortality in
Gambian infants born in the harvest season compared to those born in the hungry
season has been associated with higher amounts of IL-7 in breast milk, which
may be responsible for increased thymic index and higher sjTREC amounts in
peripheral T cells [46]. IL-7 is present in maternal milk and is capable of
crossing the gut. Il-7-deficient mice develop lymphopenia and Il-7-/-
pups when fed milk from wild type mice display increased thymic and splenic
cellularity [47].
In the first human
clinical trial for IL-7, recombinant human IL-7 upregulated Bcl2, induced
cycling and expansion of peripheral T cells including the naïve T cell
compartments and enlarged the TCR repertoire [48]. Moreover, a recombinant
human IL-7 broadened the TCR diversity and enhanced the effector memory cells
in a clinical trial consisting of patients which underwent T cell depleted
allo-HSCT [49]. Co-transduction of BM-derived mesenchymal stem cells with two
reported thymopoietic factors, IL-7 and stem cell factor has been demonstrated
to synergistically induce thymopoiesis and aid in T cell reconstitution post
BMT in mice [50]. Successful thymopoiesis as well as expansion and survival of
T cells in the periphery are observed in mice treated with IL-7 post BMT,
indicating T cell reconstitution by IL-7 is both thymus-dependent as well as
thymus-independent [51,52]. However, some contradictory results also exist. For
example, allogeneic HSCT patients with elevated IL-7 levels display increased
severity of aGVHD and reduced number lymphocytes and overall lifespan [53].
Also, blockade of the IL-7R post T cell depletion with skin allografts
diminishes cellular and humoral responses and enhances the graft survival in
mice [54].
IL-7 therapy has
been demonstrated to be harmful in HIV-infected patients. Plasma IL-7 levels
are elevated in HIV-infected patients and it is speculated to work in a
feedback mechanism to restore peripheral T cell numbers [55]. However, it is
suggested that IL-7 when administered in HIV-infected patients on HAART,
results in 70% increase in the number of circulating CD4+ T cells which contain
integrated HIV DNA. This increase in the number of T cell is not thymus-driven,
but due to enhanced T cell cycling and survival [56]. Therefore, IL-7 increases
the persistence of HIV [57]. Elevated IL-7 levels at late stages of HIV disease
progression [58] induces the expression of cell surface CXCR4 on CD4+ T cells
[55,59], resulting in a switch of HIV-1 co-receptor tropism from CCR5 to CXCR4 [60],
which may accelerate disease progression [61]. In addition, in vitro studies demonstrate
IL-7-mediated STAT5 phosphorylation and Bcl2 expression are down regulated
during HIV infection in thymocytes [62]. In corroboration, HAART in
HIV-infected patients is successful in boosting thymic functions, while
intrathymic IL-7 amounts are reduced [63], indicating an inverse correlation
between output of the thymus and IL-7 levels. Further studies are required to
fully understand the roles of IL7 as a prospective thymopoietic agent.
SEX STEROID ABLATION
Sexual dimorphism
in the thymus and thymic output has been documented in several studies. In a
couple of hypogonadal men, the numbers of naïve CD4+ T cells, i.e., CD45+CD4+
were found to be greatly increased. Importantly in these patients, the TREC
amounts reduced drastically post androgen replacement therapy [64]. One of the
plausible reasons may be the reduction in the cellularity of ETPs with age, as
it can be ameliorated by castration. Post androgen withdrawal in mice, the rise
in ETPs and eventual enhancement of thymopoiesis is mediated via increased
proliferation of TECs and production of CCL25, the CCR9 ligand, which is
crucial for ETP immigration into the thymus [65]. In male mice, cTECs are more
abundant than in females, although these cells display reduced proliferation
and low expression of FoxN1 and its target genes. In addition, the cTECs in
males express lower levels of genes crucial for thymocyte development and
selection such as Psmb11 (a cTEC-specific proteasome subunit), Ctsl (a
peptidase crucial for positive selection of CD4+ SPs) and the Notch ligand,
Delta-like 4 (Dll4) [66]. Dll4 is indispensable for T cell lineage commitment
to occur and its absence leads to emergence of immature B cells in the thymus.
Inhibition of Dll4 in cTECs by testosterone abrogates thymopoiesis in mice. Also,
chemical castration by a luteinizing hormone-releasing hormone (LHRH)
antagonist results in higher expression of Dll4 [67].
The existence of
sex-associated differences in the expression of TRA has been reported, which
culminate in higher susceptibility to autoimmune diseases in females than in
males. This is primarily due to reduced expression of AIRE in both mice and
human thymic post-puberty in females. Accordingly, castration in male mice
reduces AIRE expression [8]. Androgen recruits the androgen receptor to the
AIRE promoter regions and upregulates its transcription. This has been
demonstrated in a mouse multiple sclerosis model where androgen treatment and
the male gender confer AIRE-dependent protection against experimental
autoimmune encephalitis [68]. On the other hand, estrogen treatment
downregulates AIRE in cultured human TECs and human thymic implants in
immunodeficient mice as well as in fetal thymus organ culture. Moreover,
estrogens induce epigenetic changes in females, as the number of methylated CpG
sites on the AIRE promoter are upregulated, thus reducing AIRE expression [8].
Not surprisingly, sex
steroid inhibition has been exploited to rejuvenate the thymus. In aged
prostate cancer patients treated with
localized radiation and upon temporary chemical castration using a LHRH agonist
treatment increases the CD4+ and CD8+ T cells (naïve and memory cells) in the
periphery, along with enhanced TREC contents in the majority of the patients
[69]. Similarly, the pre-treatment of a LHRH agonist to HSCT patients augments
the naïve CD4+ T cell numbers, the diversity of the TCR repertoire and the TREC
levels in the CD4+ T cells [70].
Cancer therapies
including anti-cancer agents also lead to thymic atrophy. During chemotherapy
in testicular cancer patients, the thymus atrophies [71]. In a study, 90% of
the patients with metastatic diseases displayed reduction in volume of the
thymus during chemotherapy and its rejuvenation occurred during the recovery
phase [72]. Mice treated with anti-cancer agents such as cyclophosphamide (Cy)
develop thymic atrophy [73-75]. Cy treatment depletes the thymic CD45-
fibroblast specific protein 1 (FSP1)+ cells. The thymic FSP1+ fibroblasts in vitro release growth factors
important for TEC proliferation including FSP1, IL-6, keratinocyte growth
factor (KGF) compared to FSP1 negative cells. Mice deficient in FSP1 expression
display reduced mTECs and severe thymic atrophy [74].
OTHER THERAPIES
The thymus is
required for immune reconstitution post HAART in HIV-infected patients. Growth
hormone has been successful in increasing the density of the thymus along with
enhancing the TREC frequency in PBMCs as well as the naïve T cell counts [33].
The antioxidants, Vitamin C and N-acetyl cysteine have also demonstrated
probable thymopoietic effects by increasing the CD4+ T cell numbers along with
reducing the HIV RNA plasma levels in HIV-infected patients [76].
On the other hand,
despite showing promise as a thymopoietic agent in animal studies [14],
recombinant human KGF administration is unsuccessful in augmenting the thymic
output HIV-1 infected patients [77]. It will be worthwhile to consider the
results of the ongoing clinical trial on the effect of KGF in enhancing thymic
reconstitution and reducing the occurrences of autoimmune diseases in multiple
sclerosis patients being treated with a humanized IgG1 monoclonal antibody that
targets CD52 [78].
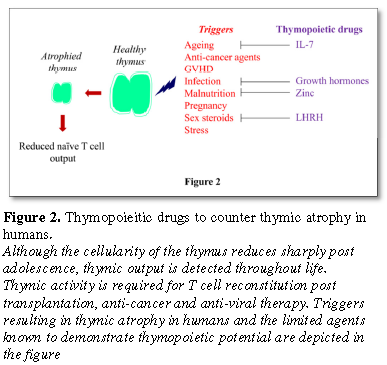
CONCLUSION
The importance of
the thymus post adolescence is vastly underappreciated. As a result, there is a
lack of studies which focus on the effect on the thymus during various ailments
and interventions to counter them. This review exclusively focuses on
conditions during which the human thymus atrophies and interventions to dampen
the process (Figure 2). Most of the studies on the thymus or its
activity are performed on animals including rodents, due to the shortage of
thymus specimens available, with the only sources being Myasthenia gravis
patients and subjects undergoing cardiac surgery [14]. Thus, the thymopoietic
drugs demonstrating potency in animal studies may not necessarily translate to
humans, such as the failure of KGF and IL-7 under certain conditions. Many
clinical trials have been performed or are ongoing which evaluate the efficacy
of drugs for thymopoietic potential. Trials such as monitoring the thymic size
and output upon androgen blockade therapy for prostate cancer in older patients
were terminated due to low accrual (NCT00379119). However, the effect of growth
hormone on the thymic function in HIV-infected adults (NCT00379119) was found
to be successful in increasing the thymic output [79]. During an antiretroviral
therapy trial, HIV-infected children were shown to interrupt the decline of
CD4+ T cells with early antiretroviral therapy. The reconstitution of CD4+ T cells
was proportional to the thymic output [80]. Some of the ongoing clinical trials
include the assessment of the efficiency of PET/CT scan and MRI to quantify the
thymic size and function (NCT02909075). A phase 2 clinical trial is active to
check for the efficacy of the androgen blocker, Lupron for immune
reconstitution, reduction in GVHD and infection in recipients of allogeneic BMT
(NCT01338987). Further studies are required which employ parameters such as
CD31 and sjTREC levels to monitor the thymic activity during various conditions
in humans. Screening and rigorously testing of molecules proven to be
thymopoietic either alone or in concert in animal models [81] can be further
considered as novel candidate therapies in humans.
DECLARATIONS
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Competing interests
The authors declare
that they have no competing interests.
Funding
Research in the
area of thymic atrophy in our laboratory is funded by SERB Grant number:
EMR/2015/002486.
Author contributions
DN conceived the
idea. SM and DN wrote and approved the final version of the manuscript.
Acknowledgment
Our studies in the
field of thymic atrophy have been possible due to grant support from SERB. We
thank DST-FIST, the DBT-IISc partnership program and UGC-SAP for providing
infrastructure support. We also thank all the members of the DpN laboratory for
their support.
1.
Geenen V, Bodart G, Henry S, Michaux H, Dardenne O,
et al. (2013) Programming of neuroendocrine self in the thymus and its defect
in the development of neuroendocrine autoimmunity. Front Neurosci 7: 187.
2.
Miller JFAP (1962) Immunological significance of the
thymus of the adult mouse. Nature 195: 1318-1319.
3.
Miller JFAP (2011) The golden anniversary of the
thymus. Nat Rev Immunol 11: 489-495.
4.
Ottaviani E, Valensin S, Franceschi C (1998) The
neuro-immunological interface in an evolutionary perspective: The dynamic
relationship between effector and recognition systems. Front Biosci 3:
d431-d435.
5.
Franchini A, Ottaviani E (1999) Immunoreactive
POMC-derived peptides and cytokines in the chicken thymus and bursa of
Fabricius microenvironments: Age-related changes. J Neuroendocrinol 11:
685-692.
6.
Bajoghli B, Guo P, Aghaallaei N, Hirano M,
Strohmeier C, et al. (2011) A thymus candidate in lampreys. Nature 470: 90-94.
7.
Guo P, Hirano M, Herrin BR, Li J, Yu C, et al.
(2009) Dual nature of the adaptive immune system in lampreys. Nature 459:
796-801.
8.
Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG,
Berthault C, et al. (2016) Estrogen-mediated down regulation of AIRE influences
sexual dimorphism in autoimmune diseases. J Clin Invest 126: 1525-1537.
9.
Takaba H, Takayanagi H (2017) The mechanisms of T
cell selection in the thymus. Trends Immunol 38: 805-816.
10.
Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R,
et al. (2004) HIV infection rapidly induces and maintains a substantial
suppression of thymocyte proliferation. Immunity 21: 757-768.
11.
Kimmig S, Przybylski GK, Schmidt CA, Laurisch K,
Möwes B, et al. (2002) Two subsets of naive T helper cells with distinct T cell
receptor excision circle content in human adult peripheral blood. J Exp Med
195: 789-94.
12.
Mitchell WA, Lang PO, Aspinall R (2010) Tracing
thymic output in older individuals. Clin Exp Immun 161: 497-503.
13.
den Braber I, Mugwagwa T, Vrisekoop N, Westera L,
Mögling R, et al. (2012) Maintenance of peripheral naive T cells is sustained
by thymus output in mice but not humans. Immunity 36: 288-297.
14.
Majumdar S, Nandi D (2017) Thymic atrophy
experimental studies and therapeutic interventions. Scand J Immunol 87: 4-14.
15.
Palmer S, Albergante L, Blackburn CC, Newman TJ
(2018) Thymic involution and rising disease incidence with age. Proc Natl Acad
Sci 115: 1883-1888.
16.
Cepeda S, Griffith AV (2018) Thymic stromal cells:
Roles in atrophy and age-associated dysfunction of the thymus. Exp Gerontol
105: 113-117.
17.
Marchetti MC, Di Marco B, Cifone G, Migliorati G,
Riccardi, C (2003) Dexamethasone-induced apoptosis of thymocytes: Role of
glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood
101: 585-593.
18.
BenjaminCL, Stowe RP, St John L, Sams CF, Mehta SK,
et al. (2016) Decreases in thymopoiesis of astronauts returning from space
flight. JCI Insight 1: e88787.
19.
Cooke KR, Luznik L, Sarantopoulos S, Hakim FT,
Jagasia M, et al. (2017) The biology of chronic graft-versus-host disease: A
task force report from the National Institutes of Health Consensus Development
Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease.
Biol Blood Marrow Transplant 23: 211-234.
20.
Dertschnig S, Nusspaumer G, Ivanek R, Hauri-Hohl MM,
Holländer GA, et al. (2013) Epithelial cytoprotection sustains ectopic
expression of tissue-restricted antigens in the thymus during murine acute
GVHD. Blood 122: 837-841.
21.
Prada N, Nasi M, Troiano L, Roat E, Pinti M, et al.
(2005) Direct analysis of thymic function in children with Down’s syndrome.
Immun Ageing 4: 1186/1742-4933-2-4.
22.
Lima FA, MoreiraFilho CA, Ramos PL, Brentani H, Lima
L de A, et al. (2011) Decreased AIRE expression and global thymic hypofunction
in Down syndrome. J Immunol 187: 3422-3430.
23.
Varga I, Bodi I, Mesanova V, Kovac M, Klein M (2018)
Association between histological alterations in the thymus and sudden infant
death syndrome. J Forensic Leg Med 55: 8-13.
24.
Goya RG, Brown OA, Pléau JM, Dardenne M (2004)
Thymulin and the neuroendocrine system. Peptides 25: 139-142.
25.
Hosea HJ, Rector ES, Taylor CG (2004) Dietary
repletion can replenish reduced T cell subset numbers and lymphoid organ weight
in zinc-deficient and energy-restricted rats. Br J Nutr 91: 741-747.
26.
Golden MH, Jackson AA, Golden BE (1977) Effect of
zinc on thymus of recently malnourished children. Lancet 2: 1057-1059.
27.
Mocchegiani E, Santarelli L, Muzzioli M, Fabris N
(1995) Reversibility of the thymic involution and of age-related peripheral
immune dysfunctions by zinc supplementation in old mice. Int J Immunopharmacol
17: 703-718.
28.
Lunin SM, Khrenov MO, Glushkova OV, Vinogradova EV,
Yashin VA, et al. (2017) Extrathymic production of thymulin induced by
oxidative stress, heat shock, apoptosis or necrosis. Int J Immunopathol
Pharmacol 30: 58-69.
29.
Fabris N, Mocchegiani E, Galli M, Irato L, Lazzarin
A (1988) AIDS, zinc deficiency and thymic hormone failure. JAMA 259: 839-840.
30.
Incefy GS, Pahwa S, Pahwa R, Sarngadharan MG, Menez
R (1986) Low circulating thymulin-like activity in children with AIDS and
AIDS-related complex. AIDS Res 2: 109-116.
31.
Mocchegiani E, Muzzioli M (2000) Therapeutic
application of zinc in human immunodeficiency virus against opportunistic
infections. J Nutr 130: 1424S-1431S.
32.
Baum MK, Lai S, Sales S, Page JB, Campa A, (2010)
Randomized, controlled clinical trial of zinc supplementation to prevent
immunological failure in HIV-infected adults. Clin Infect Dis 50: 1653-1660.
33.
Napolitano LA, Schmidt D, Gotway MB, Ameli N,
Filbert EL, et al. (2008) Growth hormone enhances thymic function in
HIV-1-infected adults. J Clin Invest 118: 1085-1098.
34.
Hansen BR, Kolte L, Haugaard SB, Dirksen C, Jensen
FK, et al. (2009) Improved thymic index, density and output in HIV-infected
patients following low-dose growth hormone therapy: A placebo controlled study.
AIDS 23: 2123-2131.
35.
Brown OA, Sosa YE, Dardenne M, Pléau J, Goya RG
(1999) Growth hormone-releasing activity of thymulin on pituitary somatotropes
is age dependent. Neuroendocrinology 69: 20-27.
36.
Iwanami N, Mateos F, Hess I, Riffel N, Soza-Ried C,
et al. (2011) Genetic evidence for an evolutionarily conserved role of IL-7
signaling in T cell development of zebrafish. J Immunol 186: 7060-7066.
37.
Ribeiro AR, Rodrigues PM, Meireles C, Di Santo JP,
Alves NL (2013) Thymocyte selection regulates the homeostasis of
IL-7-expressing thymic cortical epithelial cells in vivo. J Immunol 191: 1200-1209.
38.
Roifman CM, Zhang J, Chitayat D, Sharfe N (2000) A
partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell
development and cause severe combined immunodeficiency. Blood 96: 2803-2807.
39.
Giliani S, Mori L, de Saint Basile G, Le Deist F,
RodriguezPerez C, Forino C, et al. (2005) Interleukin-7 receptor alpha
(IL-7Ralpha) deficiency: Cellular and molecular bases. Analysis of clinical,
immunological and molecular features in 16 novel patients. Immunol Rev 203:
110-126.
40.
Silva SL, Albuquerque AS, Matoso P, Cheynier R,
Ligeiro D, et al. (2017) IL-7-induced proliferation of human naive CD4 T-cells
relies on continued thymic activity. Front Immunol 8: 20.
41.
Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S,
et al. (2013) IL-7 produced by thymic epithelial cells plays a major role in
the development of thymocytes and TCRγδ+ intraepithelial lymphocytes. J Immunol
190: 6173-6179.
42.
Alves NL, Richard-Le Goff O, Huntington ND, Sousa
AP, Ribeiro VSG, et al. (2009) Characterization of the thymic IL-7 niche in vivo. PNAS 106: 1512-1517.
43.
Okamoto Y, Douek DC, McFarland RD, Koup RA (2002)
Effects of exogenous interleukin-7 on human thymus function. Blood 99:
2851-2858.
44.
Sempowski GD, Gooding E, Liao HX Le PT, Haynes BF
(2002) T cell receptor excision circle assessment of thymopoiesis in aging
mice. Mol Immunol 38: 841-848.
45.
Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord
JM (2018) Major features of immunesenescence, including reduced thymic output,
is ameliorated by high levels of physical activity in adulthood. Aging Cell 17:
e12750.
46.
Ngom PT, Collinson AC, Pido-Lopez J, Henson SM,
Prentice AM (2004) Improved thymic function in exclusively breastfed infants is
associated with higher interleukin 7 concentrations in their mothers’ breast
milk. Am J Clin Nutr 80: 722-728.
47.
Aspinall R, Prentice AM, Ngom PT (2011) Interleukin
7 from maternal milk crosses the intestinal barrier and modulates T-cell
development in offspring. PLoS One 6: e20812.
48.
Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, et
al. (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive
T cell subsets. J Exp Med 205: 1701-1714.
49.
Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner
L, Papadopoulos EB, et al. (2012) Recombinant human interleukin-7 (CYT107)
promotes T-cell recovery after allogeneic stem cell transplantation. Blood 120:
4882-4891.
50.
Wils EJ, Rombouts EJC, van Mourik I, Spits H,
Legrand N, et al. (2011) Stem cell factor consistently improves thymopoiesis
after experimental transplantation of murine or human hematopoietic stem cells
in immunodeficient mice. J Immunol 187: 2974-2981.
51.
Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A
(2001) IL-7 increases both thymic-dependent and thymic-independent T-cell
regeneration after bone marrow transplantation. Blood 97: 1491-1497.
52.
Broers AEC, Posthumus-van Sluijs SJ, Spits H, van
der Holt B, Löwenberg B, et al. (2003) Interleukin-7 improves T-cell recovery
after experimental T-cell-depleted bone marrow transplantation in
T-cell-deficient mice by strong expansion of recent thymic emigrants. Blood
102: 1534-1540.
53.
Kielsen K, Jordan KK, Uhlving HH, Pontoppidan PL,
Shamim Z, et al. (2015) T cell reconstitution in allogeneic hematopoietic stem
cell transplantation: Prognostic significance of plasma interleukin-7. Scand J
Immunol 81: 72-80.
54.
Mai H-L, Boeffard F, Longis J, Danger R, Martinet B,
et al. (2014) IL-7 receptor blockade following T cell depletion promotes
long-term allograft survival. J Clin Invest 124: 1723-1733.
55.
Beq S, Delfraissy JF, Theze J (2004) Interleukin-7
(IL-7): Immune function, involvement in the pathogenesis of HIV infection and
therapeutic potential. Eur Cytokine Netw 15: 279-289.
56.
Lévy Y, Sereti I, Tambussi G, Routy JP, Lelièvre JD,
et al. (2012) Effects of recombinant human interleukin 7 on T-cell recovery and
thymic output in HIV-infected patients receiving antiretroviral therapy:
Results of a phase I/IIa randomized, placebo-controlled, multicenter study.
Clin Infect Dis 55: 291-300.
57.
Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB,
Sereti I, et al. (2013) Interleukin-7 promotes HIV persistence during
antiretroviral therapy. Blood 121: 4321-4329.
58.
Napolitano LA, Grant RM, Deeks SG, Schmidt D, De
Rosa SC, et al. (2001) Increased production of IL-7 accompanies HIV-1-mediated
T-cell depletion: implications for T-cell homeostasis. Nat Med 7: 73-79.
59.
Jourdan P, Vendrell JP, Huguet MF, Segondy M,
Bousquet J, et al. (2000) Cytokines and cell surface molecules independently
induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol 165:
716-724.
60.
Brieu N, Portalès P, Carles MJ, Corbeau P (2011)
Interleukin-7 induces HIV type 1 R5-to-X4 switch. Blood 117: 2073-2074.
61.
Moore JP, Kitchen SG, Pugach P, Zack JA (2004) The
CCR5 and CXCR4 co-receptors--central to understanding the transmission and
pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum
Retroviruses 20: 111-26.
62.
Young CD, Angel JB (2011) HIV infection of
thymocytes inhibits IL-7 activity without altering CD127 expression.
Retrovirology 8: 72.
63.
Ruiz-Mateos E, de la Rosa R, Franco JM,
Martinez-Moya M, Rubio A, et al. (2003) Endogenous IL-7 is associated with
increased thymic volume in adult HIV-infected patients under highly active
antiretroviral therapy. AIDS 17: 947-954.
64.
Olsen NJ, Kovacs WJ (2011) Evidence that androgens
modulate human thymic T cell output. J Investig Med 59: 32-35.
65.
Williams KM, Lucas PJ, Bare CV, Wang J, ChuYW, et
al. (2008) CCL25 increases thymopoiesis after androgen withdrawal. Blood 112:
3255-3263.
66.
Dumont-Lagacé M, St-Pierre C, Perreault C (2015) Sex
hormones have pervasive effects on thymic epithelial cells. Sci Rep 5: 12895.
67.
Velardi E, Tsai JJ, Holland AM, Wertheimer T, Yu
VWC, et al. (2014) Sex steroid blockade enhances thymopoiesis by modulating
Notch signaling. J Exp Med 211: 2341-2349.
68.
Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, et
al. (2016) Sex bias in CNS autoimmune disease mediated by androgen control of
autoimmune regulator. Nat Commun 7: 11350.
69.
Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP,
Berzins S, et al. (2005) Activation of thymic regeneration in mice and humans
following androgen blockade. J Immunol 175: 2741-2753.
70.
Sutherland JS, Spyroglou L, Muirhead JL, Heng TS,
Prieto-Hinojosa A, et al. (2008) Enhanced immune system regeneration in humans
following allogeneic or autologous hemopoietic stem cell transplantation by
temporary sex steroid blockade. Clin Cancer Res 14: 1138-1149.
71.
Hendrickx P, Döhring W (1989) Thymic atrophy and
rebound enlargement following chemotherapy for testicular cancer. Acta Radiol
30: 263-267.
72.
Choyke PL, ZemanRK, Gootenberg E, Greenberg JN,
Hoffer F (1987) Thymic atrophy and regrowth in response to chemotherapy: CT
evaluation. Am J Roentgenol 149: 269-272.
73.
Heng TSP, Goldberg GL, Gray DHD, Sutherland JS,
Chidgey AP, et al. (2005) Effects of castration on thymocyte development in two
different models of thymic involution. J Immunol 175: 2982-2993.
74.
Sun L, Sun C, Liang Z, Li H, Chen L, et al. (2015)
FSP1(+) fibroblast subpopulation is essential for the maintenance and
regeneration of medullary thymic epithelial cells. Sci Rep 5: 14871.
75.
Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno
T, et al. (2010) Sex steroid ablation enhances immune reconstitution following
cytotoxic antineoplastic therapy in young mice. J Immunol 184: 6014-6024.
76.
Müller F, Svardal AM, Nordoy I, Berge RK, Aukrust P
(2000) Virological and immunological effects of antioxidant treatment in
patients with HIV infection. Eur J Clin Invest 30: 905-914.
77.
Jacobson JM, Wang H, Bordi R, Zheng L, Gross BH, et
al. (2014) A randomized controlled trial of palifermin (recombinant human
keratinocyte growth factor) for the treatment of inadequate CD4+ T-lymphocyte
recovery in patients with HIV-1 infection on antiretroviral therapy. J AcquirImmune
Defic Syndr 66: 399-406.
78.
Keratinocyte Growth Factor - promoting thymic
reconstitution and preventing autoimmunity after alemtuzumab (Campath-1H)
treatment of multiple sclerosis. Accessed on 29 March 2019.
79.
Napolitano LA, Schmidt D, Gotway MB, Ameli N,
Filbert EL, et al. (2008) Growth hormone enhances thymic function in HIV-1
infected adults. J Clin Investig 118: 1085-1098.
80.
Lewis J, Payne H, Walker AS, Otwombe K, Gibb DM, et
al. (2017) Thymic output and CD4 T-cell reconstitution in HIV-infected children
on early and interrupted antiretroviral treatment: Evidence from the children
with HIV early antiretroviral therapy Trial. Front Immunol 8: 1162.
81.
Majumdar S, Deobagkar-Lele M, Adiga V, Raghavan A,
Wadhwa N, et al. (2017) Differential susceptibility and maturation of thymocyte
subsets during Salmonella typhimurium
infection: Insights on the roles of glucocorticoids and Interferon-gamma. Sci
Rep 7: 40793.
82.
Kumar BV, ConnorTJ, Farber DL (2018) Human T cell development,
localization and function throughout life. Immunity 48: 202-213.
83.
Patra AK, Avots A, Zahedi RP, Schüler T, Sickmann A,
et al. (2013) An alternative NFAT-activation pathway mediated by IL-7 is
critical for early thymocyte development. Nat Immun 14: 127-135.
84.
Maki K, Sunaga S, Ikuta K (1996) The V-J
recombination of T cell receptor-gamma genes is blocked in interleukin-7
receptor-deficient mice. J Exp Med 184: 2423-2427.
85.
Xiong J, Parker BL, Dalheimer SL, Yankee TM (2013)
Interleukin-7 supports survival of T-cell receptor-β-expressing CD4(-) CD8(-)
double-negative thymocytes. Immunology 138: 382-391.
86.
Patel ES, Chang LJ (2012) Synergistic effects of
interleukin-7 and pre-T cell receptor signaling in human T cell development. J
Biol Chem 287: 33826-33835.
87.
Tuulasvaara A, Vanhanen R, Baldauf HM, Puntila J,
Arstila TP (2016) Interleukin-7 promotes human regulatory T cell development at
the CD4+CD8+ double-positive thymocyte stage. JLB 100: 491-498.
88.
Brugnera E, Bhandoola A, Cibotti R, Yu Q, Guinter
TI, et al. (2000) Coreceptor reversal in the thymus: Signaled CD4+8+ thymocytes
initially terminate CD8 transcription even when differentiating into CD8+ T
cells. Immunity 13: 59-71.
89.
McCaughtry TM, Etzensperger R, Alag A, Tai X,
Kurtulus S, et al. (2012) Conditional deletion of cytokine receptor chains
reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus.
JEM 209: 2263-2276.
90.
Tani-ichi S, Shimba A, Wagatsuma K, Miyachi H,
Kitano S, et al. (2013) Interleukin-7 receptor controls development and
maturation of late stages of thymocyte subpopulations. PNAS 110: 612-617.
91.
Swainson L, Kinet S, Mongellaz C, Sourisseau M,
Henriques T (2007) IL-7-induced proliferation of recent thymic emigrants
requires activation of the PI3K pathway. Blood 109: 1034-1042.
92.
Jacobs SR, Michalek RD, Rathmell JC (2010) IL-7 is
essential for homeostatic control of T cell metabolism in vivo. J Immunol 184: 3461-3469.
93.
Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, et
al. (2012) An in vivo IL-7
requirement for peripheral Foxp3+ regulatory T cell homeostasis. J Immunol 188:
5859-5866.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Alcoholism Clinical Research
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Clinical Trials and Research (ISSN:2637-7373)