3178
Views & Citations2178
Likes & Shares
Sorafenib and sunitinib are
multiple tyrosine kinase inhibitors. Both of them have been approved by the US
FDA in the treatment of patients with malignancies. In order to develop an
effective and clinically useful chemoimmunotherapy modality against
hepatocellular cancer (HCC), we investigate their tumoricidal and immune
modulatory effect in the setting of HCC. In
vitro experiments suggested that sunitinib and sorafenib both induced HCC
cell apoptosis at an equivalent level, but stronger suppressive function to
cell proliferation was detected in sorafenib. Correspondingly, treatment of
tumor-bearing mice with sorafenib led to the suppression of tumor growth to a
larger extent than sunitinib. Flow cytometry showed that treatment with
sunitinib, not sorafenib, significantly reduced the frequency of regulatory T
cells (Tregs) and myeloid-derived suppressive cells (MDSCs) in tumor-bearing
mice; and allowed splenic lymphocytes to produce equivalent levels of IFN-γ and
TNF-α in response to vaccination as
that in wild type mice. This activation was not detected in control
and sorafenib-treated tumor mice. In addition, treatment of tumor-bearing mice
with sunitinib followed by adoptive transfer of tumor antigen-specific CD8+
T cells and immunization resulted in the additional suppression to tumor
growth compared to sunitinib monotherapy.
These results imply treatment with sunitinib, not sorafenib, is able to
prevent tumor-induced immunotolerance and activate antitumorimmunity. Our data
suggest that sunitinib may be a preferable chemotherapeutic agent
to use in combination with immunotherapy for the treatment of HCC.
Keywords:
Hepatocellular cancer (HCC), Sunitinib, Sorafenib, Chemoimmunotherapy,
Regulatory T cells (Tregs), Myeloid-derived suppressive cells (MDSCs)
Abbreviations: CCl4: Carbon Tetrachloride; CTLA-4: Cytotoxic T-Lymphocyte-Associated Protein 4; HCC: Hepatocellular Cancer; IP: Intraperitoneal; ISPL: Intra-Splenic; MDSC: Myeloid-Derived Suppressor Cell; MRI: Magnetic Resonance Imaging; PD-1: Programmed Cell Death Protein 1; PD-L1: Programmed Death-1 Ligand; RCC: Renal Cell Cancer; SOR: Sorafenib; SU: Sunitinib; TAg: SV40 T Antigen; TCR: T Cell Receptor
INTRODUCTION
Hepatocellular cancer (HCC) is a second
leading cause of cancer death worldwide [1]. The incidence and mortality of HCC
continue to increase in the United States (US) [2]. The currently available
therapeutic options only provide limited benefit [3,4]. In the last few decades
immunotherapy has become an important part of treating cancer [5]. Targeting Cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), programmed death-1 (PD-1) and programmed
death-1 ligand (PD-L1) has generated successful immunotherapeutic interventions
[6-8]. Antibodies against PD-1, CTLA-4 and PD-L1 were recently approved by the
US FDA in the treatment of patients with advanced melanoma [9] and squamous
non-small cell lung cancer et al. [10]. This clinical breakthrough encourages
the translation of immunotherapies to other cancers including HCC [3,11].
However, up to date, only few clinical trials
have been performed in patients with HCC and clinical outcome is disappointing
[12]. An intrinsic immune suppressive microenvironment represents a major
impediment [4]. One promising immune-based therapeutic modality of HCC is
chemoimmunotherapy [13] in which chemotherapy not only exerts inherent tumoricidal
effect but also restores the ability of immune system to destroy the
established tumors [13,14]. In the present study, we compare the role of
FDA-approved chemotherapeutic drugs sunitinib [13,14] and sorafenib [15] in
overcoming tumor-induced immunotolerance and synergizing with immunotherapy in
the treatment of HCC.
Sorafenib (Bayer Pharmaceuticals, West Haven, CT) and sunitinib (Pfizer Inc., New York, NY) are small molecular inhibitors of multiple tyrosine kinases. Both of them displaying similar drug profiles and overlapping targets, have been approved by the US FDA for advanced renal cell cancer (RCC) [16,17]. In 2008, sorafenib became the first and only systemically administered therapy for unresectable HCC, as it increases the median overall survival of patients from 7.9 to 10.7 months [18]. In 2013, one group conducted an open-label, phase III trial to compare the therapeutic effect of sunitinib and sorafenibin HCC. The results indicated the overall survival with sunitinib was not superior or equivalent to sorafenib [19]. With the development of immunotherapy over the past several years, evaluating the effect of sunitinib and sorafenib in antitumor immunity in the context of HCC towards development of curative chemoimmunotherapy has gained increasing interest. Using new clinically relevant murine model established recently by us, we assess the role of sunitinib and sorafenib in antitumor immunity in the setting of HCC and investigate each monotherapy and the combination with adoptive transfer of tumor antigen-specific CD8+ T cells in the treatment of HCC.
MATERIALS AND METHODS
Cell line, cell proliferation and apoptosis assay
Human hepatocellular carcinoma cell line HepG2
and human hepatoma cell line SkHep1 were obtained from American Type Culture
Collection (Manassas, VA) and grown in MEM with 10% FBS at 37°C in 5% CO2 humidified
atmosphere. B6/WT-19 cell is a transformed C57BL/6 mouse embryofibroblast line
that expresses wild-type SV40 T antigen (TAg). 2 × 104 Sk-Hep1 or
HepG2 cells were seeded into each well of 96-well plate then treated with the
indicated concentrations of sunitinib or sorafenib. Cell proliferation and
apoptosis assays at the indicated time were conducted with the Proliferation
Assay Kit (Promega) and Apo-one Homogeneous Caspase-3/7 Assay kit (Promega)
according to the manufacturer’s instructions.
Mice
Line MTD2 [20] and 416 [21] mice served as the
source of tumorigenic hepatocytes and tumor antigen-specific (TAS) CD8+
T cells (TCR-I T cells), respectively. Male C57BL/6 mice from Jackson
Laboratory (Bar Harbor, ME) were used as recipient mice in our HCC model. Animal experiments were approved by the ACUC
of University of Missouri-Columbia. All mice received humane care according to the
criteria outlined in the “Guide for the Care and Use of Laboratory Animals”.
IP
administration of CCl4, ISPL injection of hepatocytes and magnetic
resonance imaging (MRI)
10% CCl4 (v/v) solution in corn oil
was intraperitoneally (IP) injected into C57BL/6 mice twice a week at 8 mL/kg
of body weight (BW) for six weeks. Two weeks after last injection, the mice
received TAg-transgenic hepatocytes isolated from young male MTD2 mice by
intrasplenic (ISPL) injection [13]. Briefly, C57BL/6 mice under general anesthesia
underwent a ½ cm flank incision. Two 10 mm titanium clips were placed between
the upper and lower branch of the splenic vasculature and spleen was cut
between the two clips. Hepatocytes were injected into the lower pole of the
spleen. The lower pole of the spleen was removed following injection. All MRI scans for tumor surveillance were performed on a 7 Tesla AVANCE
III BioSpec system equipped with a 35 mm quadrupture detection radiofrequency
coil (Bruker BioSpin, Billerica, MA). Tumor images were obtained using a
respiratory-gated multi-slice T2-weighted sequence, with an in-plane resolution
0.1 × 0.15 mm and slice thickness 1 mm.
In vivo
treatment of tumor-bearing mice with sunitinib or sorafenib and immunization
with B6/WT-19 cells
Sunitinib was orally administrated to each
mouse at 40 mg/kg of BW in 0.2 mL every other day for two weeks. Sorafenib was
orally administrated to each mouse at 30 mg/ml daily for 2 weeks. For
immunization, 3 × 107 B6/WT-19 cells freshly harvested were suspended
in 0.2 mL of PBS and IP injected into each mouse [13].
Isolation and purification of TCR-I
transgenic T cells and the adoptive transfer
416 mice is a transgenic strain carrying a
rearranged TCR transgene specific for the H2-Db-restricted TAg epitope I
(residues 206-215: SAINNYAQKL). These mice are now available from the Jackson
Laboratory as line B6.Cg-Tg (TcraY1, TcrbY1) 416Tev/J. Transgene positive TCR-I
progenies were identified by staining peripheral blood lymphocytes with
FITC-labeled anti-Vβ7 antibody (BD Pharmingen). In the present studies, 12 week
old 416 mice were euthanized to isolate spleen or lymph nodes for isolating
lymphocytes. CD8+ TCR-I T cells were enriched by MACS sorting using
CD8+ magnetic microbeads (Miltenyi Biotech, Auburn, CA) according to
the manufacturer’s instructions. CD8-enriched cells were stained with anti-CD8
and Db/I tetramer to determine purity, which ranged between 85-90%. 1 × 106
purified TCR-I T cells were suspended in 0.2 mL of HBSS and injected into the
mice via tail vein.
Flow cytometric analysis
Ex vivo staining of splenic
lymphocytes with fluorochrome-labeled antibodies was performed on single-cell
suspensions [14]. Stained cells were analyzed with a FACScan flow cytometer (BD
Biosciences). Data were analyzed using FlowJo software (Tree Star). Staining
for intracellular IFN-γ and TNF-α was performed as described previously [13].
Staining for FoxP3 was performed with a buffer set from eBioscience.
Statistics
Paired data were analyzed using a 2-tailed
paired Student’s t test. A p value of less than 0.05 was
considered significant.
RESULTS
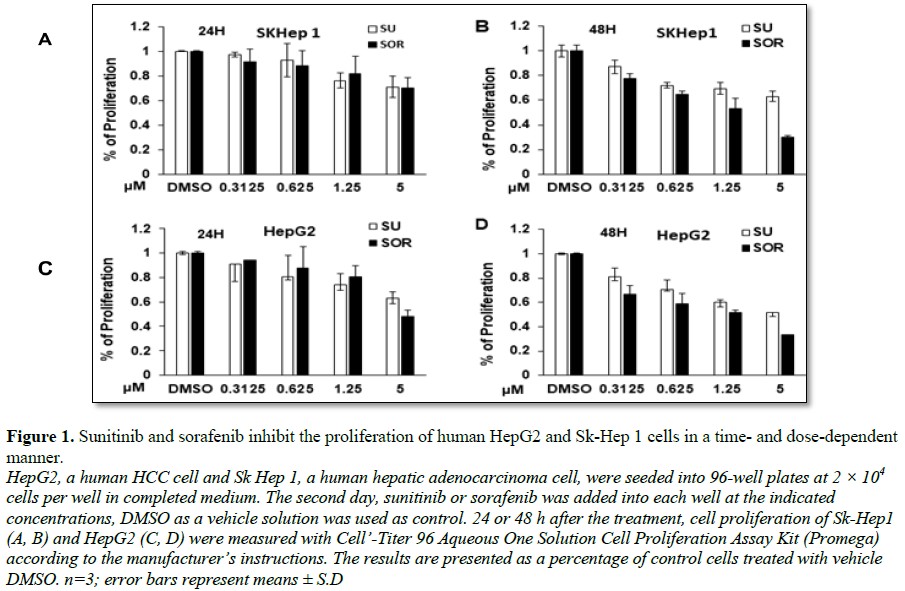
Sunitinib and sorafenib suppress HCC and hepatoma cell growth in vitro
To compare the cytotoxic property of sunitinib
and sorafenib in HCC and hepatoma cells, 2 × 104 cells were seeded
and cultured in each well of 96-well plate. 24 or 48 hours post treatment,
proliferation and apoptosis of the cells with indicated treatments were
measured. The results were presented as the percentage of cells undergoing
proliferation in comparison with control without
treatment. As shown in Figures 1A-1D,
the results indicated that sunitinib and sorafenib both inhibited the
proliferation of two types of cells in a dose- and time-dependent manner. More
suppressive effect was observed in HepG2 cells compared to Sk-Hep1 cells. For
example, 5 µM sunitinib or sorafenib treatment for 24 h led to the reduction of
Sk-Hep1 cells to about 70%, however approximately 60% and even less
proliferation were detected in HepG2 cells. Compared to sunitinib, sorafenib
exerted more cytotoxic effect on these two cell types. For example, treatment
of Sk-Hep1 or HepG2 with 5 µM sunitinib or sorafenib for 48 h, about 60% or 50%
proliferated cells were detected (Figure
1B). In contrast, only less than 30% proliferated cells were detected in
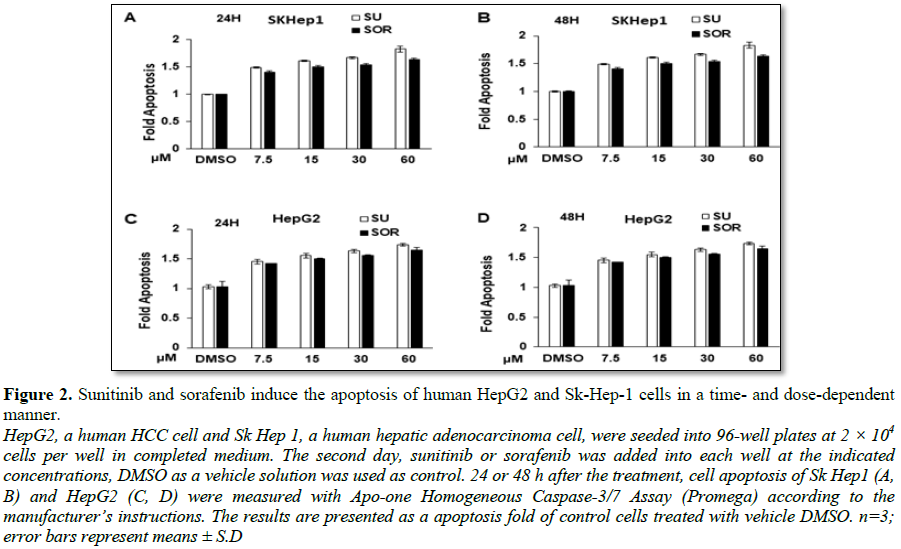
HepG2 and Sk-Hep1 cells treated with sorafenib (Figure 1D). Correspondingly, treatment with the two
chemotherapeutic drugs induced cell apoptosis in Sk-Hep1 and HepG2 cells in the
time- and dose-dependent ways (Figures
2A-2D). The extent of induced apoptosis with these two drugs was very
similar, no much difference of cytotoxic effect was observed between Sk-Hep1
cells and HepG2 cells. Together, FDA-approved sunitinib and sorafenib similarly
exert cytotoxic activity on HCC cells.
Sunitinib
and sorafenib treatment resulting in frequency alteration of immune cell subsets
in tumor-bearing mice
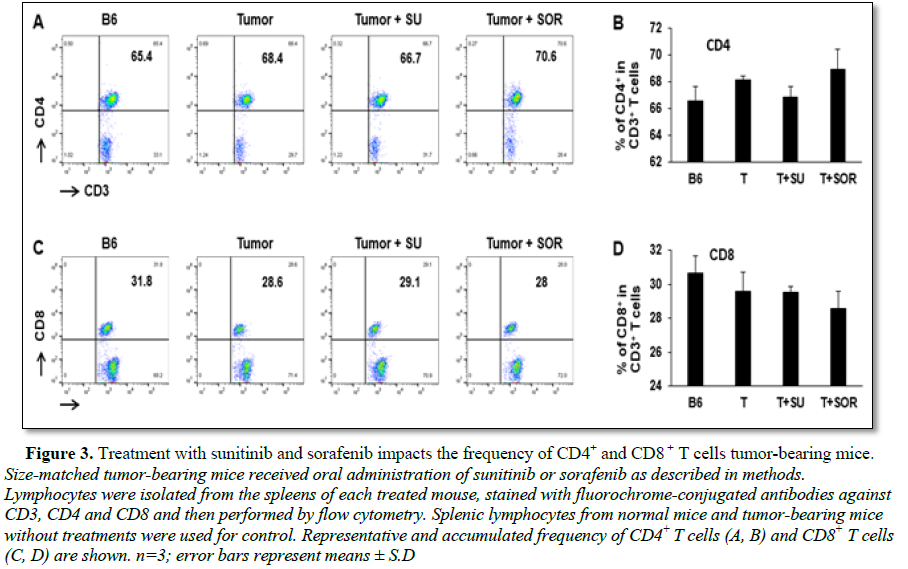
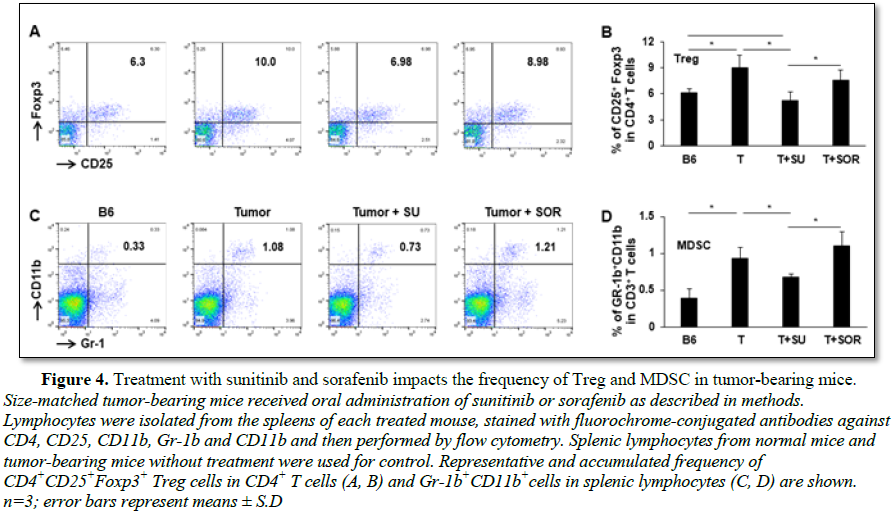
In addition to the cytotoxic effect on tumor cells, we also explored and compared the role of sunitinib and sorafenib on immune system in tumor-bearing mice. First, we investigated whether sunitinib and sorafenib treatments differently modulate the frequencies of immune cell populations. Comparing control tumor-bearing mice without treatment, sunitinib administration led to the slight reduction in the frequency of CD4+ T cells from 68% to 67% (Figures 3A and 3B), but small increase of CD4+ T cells seen in sorafenib-treated mice from 68% to 70% (Figures 3C and 3D). No effect on frequency of CD8+ T cells was detected in either sunitinib- or sorafenib-treatment. Conversely, sunitinib treatment significantly reduced the magnitude of Tregs (CD4+CD25+FoxP3+) from 10% in control tumor-bearing mice without treatment to 7% (Figures 4A and 4B), and MDSCs (CD11b+Gr-1+) from 1.1% to 0.7% (Figures 4C and 4D). Sorafenib treatment led to the slight reduction of Treg frequency and small increase of MDSCs. Both changes were not statistically significant. These results suggest that sunitinib treatment reduces the frequency of immunosuppressive cell populations in the setting of HCC.
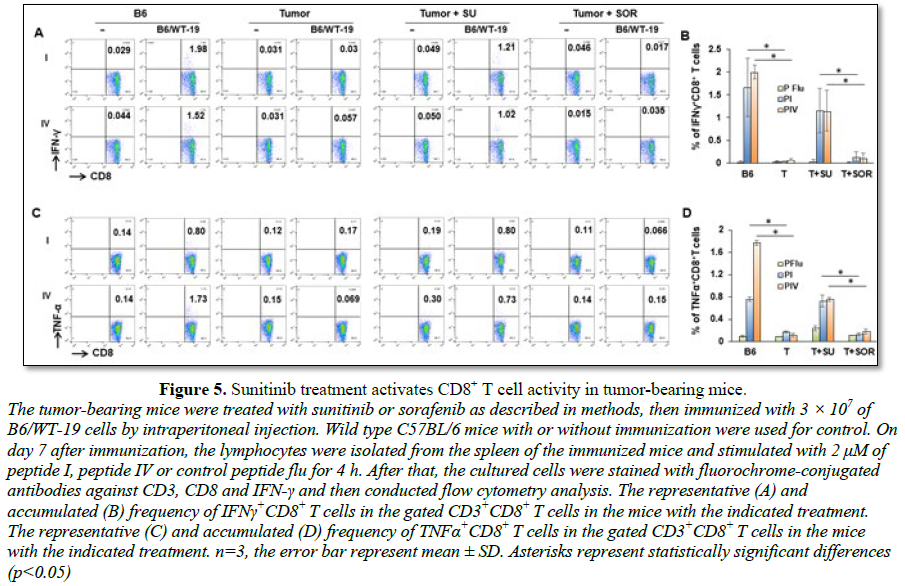
Sunitinib
and sorafenib treatment impact effector CD8+ T cell activity
To investigate if treatment with sunitinib or
sorafenib is able to improve antitumor function, tumor-bearing mice were
divided into three groups and receive vehicle, sunitinib and sorafenib
treatment, respectively. Following the indicated treatments, half of mice in
each group received tumor antigen-specific immunization with transgenic
B6/WT-19 cells expressing TAg. Wild type mice with or without immunization were
used for control. Splenic lymphocytes were isolated from each mouse seven days
post immunization and were stimulated with TAg epitope-I or -IV. The resultant
production of IFN-γ and TNF-α in effector CD8+ T cells were
measured with flow cytometry. As shown in Figure
5, epitope-I and epitope-IV were both unable to stimulate the production of
IFN-γ and TNF-α in effector CD8+ T cells in vehicle-
and sorafenib-treated tumor-bearing mice no matter whether they received
immunization and not. Conversely, sunitinib treatment activated CD8+ T
cells from immunized tumor-bearing mice, allowing epitope-I and -IV to effectively
stimulate CD8+ T cells producing IFN-γ and TNF-α. The levels were equivalent to that seen in the
immunized wild type mice. These results suggest that sunitinib treatment
restores the activity of effector CD8+ T cells in HCC.
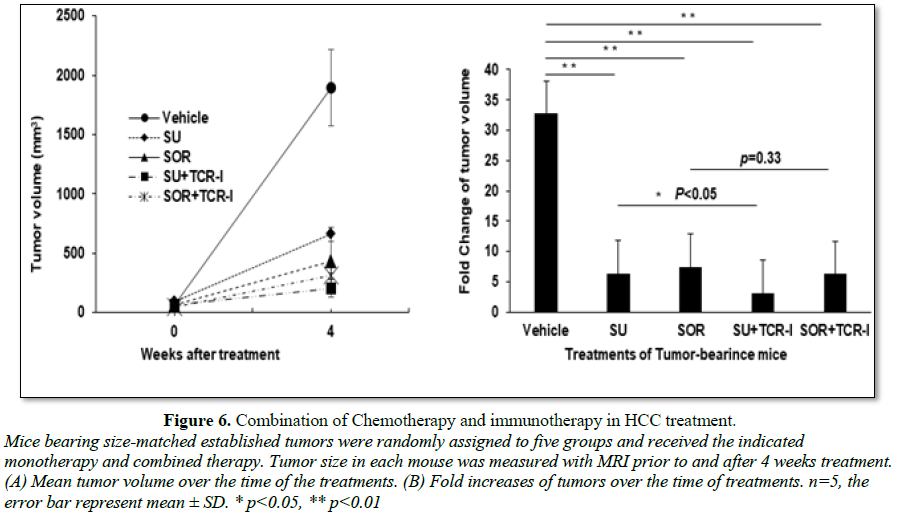
Combination
of sunitinib or sorafenib with TCR-I T cells plus immunization in the treatment
of HCC
To further investigate combination of
chemotherapy and immunotherapy in the treatment of HCC, size-matched
tumor-bearing mice were divided into five groups and received the following
treatments: vehicle control, sunitinib monotherapy, sorafenib monotherapy,
sunitinib or sorafenib combination with TCR-I T cells. All of mice were given
the tumor antigen-specific immunization with transgenic B6/WT-19 cells
expressing TAg. Four weeks after initial treatment, the tumor volume in each
mouse was measured with MRI. We found that sunitinib and sorafenib
monotherapies and their combination therapies effectively suppressed tumor
growth. On week 4 after treatment, the mean tumor volumes were about 650 mm3,
430 mm3, 200 mm3 and 310 mm3 in each indicated
group which were much less than 1800 mm3 seen in vehicle control
mice (Figure 6A). The fold increase
of tumor volume is 6.4, 7.4, 3.1, 6.2, respectively; all of them were much less
than 32.7 fold seen in vehicle control group (Figure 6B). We observed addition of immunotherapy with TCR-I T
cells and immunization to sunitinib monotherapy led to further suppression to
tumor growth; but only minor effect was detected in sorfenib combination
treatment. Together, combination of sunitinib and adoptive transfer of tumor
antigen-specific CD8+ T cells is demonstrated to be an effective
chemoimmunotherapic strategy, preventing HCC progression.
DISCUSSION
In the present study, we compare the cytotoxic
characteristic and immune modulatory effect between sunitinib and sorafenib in
the context of HCC. Both of them maintain capability to inhibit growth of HCC
cells and tumors. Interestingly, sunitinib shows a very strong immune
modulatory effect in our clinically relevant murine model. As a result, combination
of sunitinib treatment with external tumor antigen-specific CD8+ T
cells plus immunization significantly suppresses tumor progression (Figure 6). This effect is not detected
in mice receiving sorafenib-integrated treatment. These synergistic results
emphasize the combination of sunitinib with immunotherapy have a therapeutic
potential in the treatment of HCC and sunitinib functions as an effective
immune adjuvant to boost antitumor immune response.
Our findings demonstrate that’ sunitinib may be
a preferable chemotherapeutic agent to use in combination with immunotherapy
for the treatment of HCC [22]. While sunitinib and sorafenib have similar
structure and tumoricidal effect, their effect on immune system is obviously
different. We demonstrate that sunitinib treatment results in the significant
reduction in the frequency of Treg and MDSC (Figure 4), allowing activation of endogenous effector CD8+ T
cells in response to the immunization with tumor specific antigens. The results
support previous findings in several clinical trials. Brossart’s group [23]
found that sorafenib, but not sunitinib, inhibits function of DCs, impaired
DC’s ability to migrate and stimulate T-cell responses. In contrast, sunitinib
treatment reduced regulatory T cells in peripheral blood mononuclear cells. Van
Herpen [24] enrolled 40 subjects in their clinical trial. 16 RCC patients were
treated with sunitinib, 6 patients with sorafenib, 7 non treated controls and
11 healthy controls. Although all patients receiving sunitinib or sorafenib
developed seroprotection to influenza vaccination comparable with controls,
functional T-cell activity was only observed in three groups, rather than
patients treated with sorafenib, evidenced by a decreased proliferation rate
and IFN-γ/IL-2 production and increased IL-10 level compared with healthy
controls. Salih’s group reported that pharmacological concentrations of sorafenib,
but not sunitinib, inhibited cytotoxicity and cytokine production of resting
and IL-2-activated PBMC [25], as sorafenib impaired reactivity of NK cells. NK
cells substantially contribute to antitumor immunity by directly killing target
cells and shaping adaptive immune responses through secreting cytokines like
IFN-γ. These data suggest that sunitinib is able to activate immune response by
modulating different immune cell subsets. In contrast, Perez-Gracia et al. [26]
reported that sorafenib was able to block VEGF-mediated impairment on DCs
derived from normal persons through inhibiting its differentiation and
maturation; however, this effect was not seen in sunitinib. These data imply
that sunitinib might only modulate DCs from patients.
Our studies also support findings from an
open-label, phase III study which compares sunitinib versus sorafenib in
advanced HCC [19]. The investigators reported that sorafenib may be safer and
more effective than sunitinib as a monotherapy. We demonstrated that in vivo treatment of tumor-bearing mice
with sunitinib and sorafenib monotherapy at same concentrations slowed down
tumor growth with stronger effect seen in sorafenib (Figure 6). In vitro
experiments suggested that this effect was mediated by suppressing tumor cell
proliferation (Figure 1) and
inducing tumor cell apoptosis (Figure 2).
While the efficacy of inducing apoptosis with sunitinib and sorafenib was
similar, more suppressive effect on HCC cell proliferation was detected in
sorafenib.
In summary, sunitinib and sorafenib, as
FDA-approved chemotherapeutic agents, differently impact antitumor immunity in
the setting of HCC. Pretreatment of tumor bearing mice with sunitinib is able
to prevent tumor-induced immunotolerance, activating tumor antigen-specific T
cells to suppress tumor growth. Thus, integration of sunitinib and
immunotherapy may be an effective therapeutic modality which can be translated
into clinical practice of HCC. We will apply for a clinical trial to explore
sunitinib-immunotherapy regimens in the treatment of patients with HCC and
elucidate the underlying mechanisms.
ACKNOWLEDGEMENT
The authors thank Jeremy Haley for expert
technical assistance and Harry S Truman Memorial VA
Hospital Biomolecular Imaging Center for measuring tumor size with MRI.
COMPETING
FINANCIAL INTERESTS
The authors have declared that no conflict of
interest exists.
FINANCIAL
SUPPORT
Grant Support: R01 CA164335-01A1 (Kevin F
Staveley-O’Carroll PI), R01CA208396 (Kevin F
Staveley-O'Carroll, Guangfu Li, Mark Kester) from the National
Cancer Institute/National Institutes of Health.
1.
El-Serag HB, Kanwal F (2014) Epidemiology of
hepatocellular carcinoma in the United States: Where are we? Where do we go?
Hepatol 60: 1767-1775.
2.
White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB
(2017) Incidence of hepatocellular carcinoma in All 50 United States, from 2000
through 2012. Gastroenterol 152: 812-820.
3.
Liu D, Staveley-O'Carroll KF, Li G (2015) Immune-based
therapy clinical trials in hepatocellular carcinoma. J Clin Cell Immunol 6.
4.
Tagliamonte M, Petrizzo A, Tornesello ML, Ciliberto G,
Buonaguro FM, et al. (2016) Combinatorial immunotherapy strategies for hepatocellular
carcinoma. Curr Opin Immunol 39: 103-113.
5.
Sathyanarayanan V, Neelapu SS (2015) Cancer
immunotherapy: Strategies for personalization and combinatorial approaches. Mol
Oncol 9: 2043-2053.
6.
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint
blockade in cancer therapy. J Clin Oncol 33: 1974-1982.
7.
Topalian SL, Drake CG, Pardoll DM (2015) Immune
checkpoint blockade: A common denominator approach to cancer therapy. Cancer
Cell 27: 450-461.
8.
Dart A (2016) Immunotherapy: Checkpoint barriers. Nat
Rev Cancer 16: 678.
9.
(2015) Pembrolizumab superior to ipilimumab in
melanoma. Cancer Discov 5: 568.
10.
Waqar SN, Morgensztern D (2015) Immunotherapy for
non-small cell lung cancer: Are we on the cusp of a new era? Expert Rev Clin
Immunol 11: 871-873.
11.
Li G, Staveley-O'Carroll KF, Kimchi ET (2016)
Potential of radiofrequency ablation in combination with immunotherapy in the
treatment of hepatocellular carcinoma. J Clin Trials 6.
12.
Sprinzl MF, Galle PR (2017) Current progress in
immunotherapy of hepatocellular carcinoma. J Hepatol 66: 482-484.
13.
Li G, Liu D, Cooper TK, Kimchi ET, Qi X, et al. (2016)
Successful chemoimmunotherapy against hepatocellular cancer in a novel murine
model. J Hepatol.
14.
Avella DM, Li G, Schell TD, Liu D, Zhang SS, et al.
(2012) Regression of established hepatocellular carcinoma is induced by chemoimmunotherapy
in an orthotopic murine model. Hepatol 55: 141-52.
15.
Porta C, Paglino C, Imarisio I, Ganini C, Pedrazzoli P
(2011) Immunological effects of multikinase inhibitors for kidney cancer: A clue
for integration with cellular therapies? J Cancer 2: 333-338.
16.
Chow LQ, Eckhardt SG (2007) Sunitinib: From rational
design to clinical efficacy. J Clin Oncol 25: 884-896.
17.
Grandinetti CA, Goldspiel BR (2007) Sorafenib and
sunitinib: Novel targeted therapies for renal cell cancer. Pharmacotherapy 27:
1125-1144.
18.
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS,
Lencioni R, et al. (2008) Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst 100: 698-711.
19.
Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, et al.
(2013) Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of
a randomized phase III trial. J Clin Oncol 31: 4067-4075.
20.
Held WA, Mullins JJ, Kuhn NJ, Gallagher JF, Gu GD, et
al. (1989) T antigen expression and tumorigenesis in transgenic mice containing
a mouse major urinary protein/SV40 T antigen hybrid gene. EMBO J 8: 183-91.
21.
Staveley-O'Carroll K, Schell TD, Jimenez M, Mylin LM,
Tevethia MJ, et al. (2003) In vivo
ligation of CD40 enhances priming against the endogenous tumor antigen and
promotes CD8+ T cell effector function in SV40 T antigen transgenic mice. J
Immunol 171: 697-707.
22.
Seliger B, Massa C, Rini B, Ko J, Finke J (2010) Anti-tumor
and immune-adjuvant activities of protein-tyrosine kinase inhibitors. Trends
Mol Med 16: 184-92.
23.
Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, et al.
(2008) Sorafenib, but not sunitinib, affects function of dendritic cells and
induction of primary immune responses. Blood 111: 5610-20.
24.
Mulder SF, Jacobs JF, Olde Nordkamp MA, Galama JM,
Desar IM, et al. (2011) Cancer patients treated with sunitinib or sorafenib
have sufficient antibody and cellular immune responses to warrant influenza
vaccination. Clin Cancer Res 17: 4541-4549.
25.
Krusch M, Salih J, Schlicke M, Baessler T, Kampa KM,
et al. (2009) The kinase inhibitors sunitinib and sorafenib differentially
affect NK cell antitumor reactivity in vitro. J Immunol 183: 8286-8294.
26.
Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, et
al. (2009) Influence of bevacizumab, sunitinib and sorafenib as single agents
or in combination on the inhibitory effects of VEGF on human dendritic cell
differentiation from monocytes. Br J Cancer 100: 1111-1119.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Dermatology Clinics and Research (ISSN:2380-5609)