992
Views & Citations10
Likes & Shares
Aim: To investigate the
protective effect of 20-hydroxyecdysterone (20E) against
lipopolysaccharide-induced acute lung injury (ALI) in mice.
Materials and
methods: Mice were respectively administrated a single intratracheal
instillation with normal saline (normal group) and 4 mg/kg lipopolysaccharide
(LPS) (LPS cohort). Next, animals of LPS cohort were subsequently divided into
the model group, the control group, the low-dose group of 20E, middle-group of
20E and high-dose group of 20E. Histological changes of lung were examined by
hematoxylin and eosin. Expression levels of tumor necrosis factor-α (TNF-α),
interleukin-2 (IL-2), IL-6, IL-8, IL-4 and IL-10 were determined by real-time
PCR and enzyme-linked immuno-sorbent assay, respectively.
Results: 20E treatment could
result in a decrease of lung damage in the ALI mice. In the 20E treated groups,
expression of TNF-α, IL-2, IL-6 and IL-8 was significantly inhibited compared
with that model group, respectively. In addition, expression of IL-4 and IL-10
was induced in the 20E treated groups, respectively.
Conclusion: The results suggest
that 20E play a protective-role in ALI of mice probably by inhibiting the
pro-inflammory cytokine expression and enhancing the anti-inflammatory cytokine
expression.
Keywords:
20-hydroxyecdysterone, Acute lung injury, Protective effects, Pro-inflammation
cytokines, Anti-inflammatory cytokines
INTRODUCTION
Acute lung injury (ALI) is
considered as a major health problem for elderly population and characterized
by activation of the pulmonary endothelium, disruption of the endothelial and
alveolar epithelial barriers, and increase of the microvascular permeability
[1]. Lung, a crucial airway for pathogens into the body, is widely contacted
with a large number of micro-organisms those can cause acute inflammation and
ALI [2]. Evidences have demonstrated that that inflammation is closely
associated with the occurrence of ALI. In the inflammation, over-production of
inflammatory cytokines are involved into injury of lung tissues [3,4].
Lipopolysaccharides (LPS) presented in cell wall of gram-negative bacterium can
cause ALI [5]. Lung injury induced LPS is close link with release of
macrophage-derived pro-inflammatory cytokines as well as anti-inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-2,
IL-6, IL-8, IL-4, IL-10 and interferon-γ [6,7].
Ecdysterone
was first isolated from insect and plays a key role in the molting, development
and reproduction of animals. Afterward, it was confirmed that the active
component of 20-hydroxyecdysterone (20E) is widespread across the plant
kingdom and sustain higher concentration in them [8,9]. It has demonstrated
that 20E have remarkable pharmacological properties in mammals, including
lowering cholesterol levels and blood glucose, stimulating protein synthesis,
promoting carbohydrate and lipid metabolism, and inducing stem cell
differentiation [10]. Recently, it has been suggested that 20E has obvious
antioxidant activity by radical scavenging tests in vitro and in vivo
[11]. Meanwhile, our study indicates that 20E can function as an important
player in counteracting memory deficits in rats with diabetes, possibly through
enhancing the anti-oxidative ability in the brain [12]. But, little is known
about the effect of 20E on the ALI. Thus, the current study was designed to shed
light of the effect of 20E on the ALI.
MATERIALS AND METHODS
Reagents
The reagents used in the experiment and their
sources were as follows: lipopolysaccharide (LPS) and 20E were obtained from
Sigma-Aldrich Co (St. Louis, MO, USA), dexamethasone-21-acetate (Dex) and
sodium pentobarbital were purchased from Aladdin (Shanghai, China). PCR primers
were synthesized by Sangon Biological Engineering Technology Company (Shanghai
China). Trizol reagent and RT-PCR kit including RTase M-MLV, dNTP mixture, Taq
DNA polymerase, RNase inhibitor and SYBR Premix Ex TaqTM were obtained from
Takara (Dalian, China). Enzyme-linked
immuno-sorbent assay (ELISA) kit of mice was purchased from BOSTER (Wuhan,
China). All other chemicals were of reagent grade.
Animals
The experimental study was performed following
approval from the Pingdingshan University Animal Care and Ethics Committee. All
experimental and surgical procedures were conducted by the Pingdingshan
University Surgical Application and Research Center. Animal cultures were
performed according to the National Institutes of Health Guide for the Care and
Use of Laboratory Animals. Male ICR mice (20-24 g) were obtained from Henan
Laboratory Animals Center for Medical Science and Research. The animals were
fed standard laboratory chow (Wuhan University Center for Animal experiment,
Wuhan, China) with free access to water and housed individually at a controlled
temperature of 19-25℃ with a 12:12 h light/dark cycle.
Animals were randomly divided in six groups (n=15)
including normal group, model group, control group (Dex group), low-dose group
of 20E, middle-dose group of 20E and high-dose group of 20E. Except normal
group, mice in other groups were anaesthetized with an intraperitoneal
injection of 40 mg/kg of sodium pentobarbital and treated with a single
intratracheal instillation with 4 mg/kg LPS. Based on previous study [12],
animals in the low-dose group of 20E, middle-dose group of 20E and high-dose
group of 20E received 20E solution at a concentration of 0.1 mg/kg, 1 mg/kg and
10 mg/kg, respectively, and Dex group received Dex at 10 mg/kg. Times of animal
treatment were 1, 12, 24 and 36 h before LPS instillation and 12, 24 and 36 h
after LPS challenge. Animals from normal group and model group were treated
with the same volume of normal saline at corresponding times. The mice were
killed by cervical dislocation. Left and right part of lungs were separated,
harvested, immediately frozen in liquid nitrogen, and then stored at -80℃ until
used.
Histological analysis of lung
Left lungs were fixed in 10% formaldehyde and
embedded in paraffin. Specimens were cut with 4 μm thick sections those were
stained by routine hematoxylin and eosin (H&E) for histological analysis.
Sections were analyzed under light microscope (Olympus BX50, Barcelona, Spain).
Real-time PCR assay for TNF-α, IL-2, IL-6, IL-8, IL-4 and IL-10 mRNA
level in the lung
Total RNA was extracted from lung tissues using the
Trizol reagent according to the manufacturer’s protocol. Quality of RNA was
monitored by 1.2% agarose gel electrophoresis. First-strand cDNA was
synthesized using M-MLV reverse transcriptase.
ELISA assay for the TNF-α, IL-2, IL-6, IL-8, IL4 and IL10 protein levels
in the lung
Right lungs were weighted, perfused with chilled
normal saline, then cut into small pieces, placed in 0.2 M phosphate buffer (pH
7.4) and homogenized using homogenizer to obtain 20% homogenate. Resultant
homogenate was centrifuged (5000x g for 15 min, 4℃) and the supernatant was sub
packaged to sterile eppendorf tubes. Levels of TNF-α, IL-2, IL-6, IL-8, IL-4
and IL-10 in lung were measured by ELISA kits. All the procedures were
performed in accordance with the manufacturer’s instructions.
Statistical analysis
Original data were analyzed by Statistical Package
for Social Sciences software (version 14.0) (SPSS, Inc., Chicago, IL). Results
were expressed as mean ± SEM. Multiple sets of data comparison were carried out
by one-way analysis of variance (ANOVA). Comparison of data between groups were
treated by least significance difference (LSD) combined with Tamhane’s way.
Values with P<0.05 were considered as statistically significant.
RESULTS
Histopathologic analysis
Histopathological examination of normal animals
showed normal cellular architecture with distinct alveolar cell (Figure 1A).
The lung sections of model group exhibited an obvious damage characterized by
focal hemorrhage, distortion and alveolar thickening (Figure 1B).
Compared with that of model group, injury derived from LPS was ameliorated by
Dex treatment and different-dose 20E treatment (Figures 1C-1F).
Furthermore, obvious protecting-role was observed in Dex and high-dose group of
20E (Figures 1C and 1F).
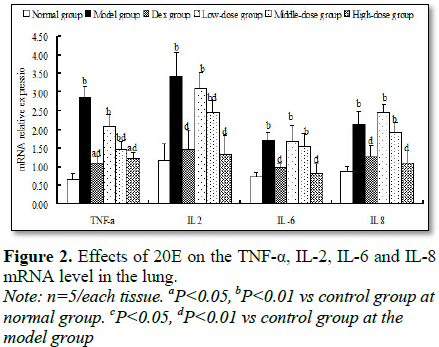
Effects of 20E on the TNF-α, IL-2, IL-6, IL-8, IL-4 and IL-10 mRNA level
in the lung
Compared with that of normal
group and Dex group, the mRNA levels of TNF-α, IL-2, IL-6 and IL-8 were
significantly increase in the model group (Figure 2). Administration of
different-dose of 20E could result in a dose-dependent decline of TNF-α, IL-2,
IL-6 and IL-8 level. In the high-dose group of 20E, mRNA level of TNF-α, IL-2,
IL-6 and IL-8 decreased 58.1% (P<0.01), 61.1% (P<0.01), 52.1% (P<0.01)
and 47.7% (P<0.01) compared with that of model group (Figure 2),
respectively.
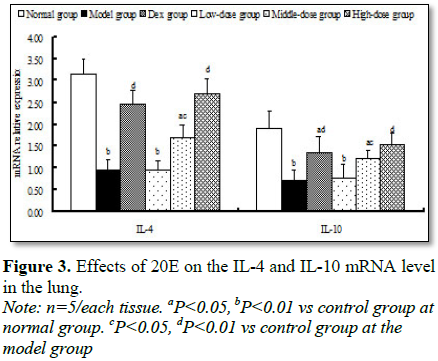
Compared with that of
normal group and Dex group, mRNA levels of IL-4 and IL-10 were significantly
decreased in the model group (Figure 3). But, in high-dose group of 20E,
mRNA level of IL-4 and IL-10 increased 80.6% (P<0.01) and 116.8% (P<0.01)
in contrast with that of model group, respectively (Figure 3).
Effects of 20E on the TNF-α, IL-2, IL-6, IL-8, IL-4 and IL-10 expression
in the lung
Compared with that of normal group and Dex group, in
a significant increase of TNF-α, IL-2, IL-6 and IL-8 level was observed in the
model group (P<0.01) (Table 2). Expression levels of TNF-α, IL-2,
IL-6 and IL-8 showed a decline trend in different 20E treated groups compared
with that of model group. In the high-dose group of 20E, level of TNF-α, IL-2,
IL-6 and IL-8, respectively decreased 48.3% (P<0.01), 66.3% (P<0.01),
26.9% (P<0.05) and 45.7% (P<0.01) (Table 2), but the level of IL-4 and
IL-10 increased 1.9 times (P<0.01) and 2.9 times (P<0.01) in contrasted
with that of model group (Table 2).
DISCUSSION
In the current study, administration of 20E resulted
in a decrease of the alveolar cell damage and decline of the TNF-α, IL-2, IL-6
and IL-8 expression in the ALI mice, which suggested a protective-role of 20E
against LPS injury is closely associated with suppression of TNF-α, IL-2, IL-6
and IL-8 production. Inflammation cascade is initiated by the innate immune
system, in which TNF-α, IL-2, IL-6 and IL-8 play a key role in the inflammatory
response [13]. Generally, inflammatory response is taken as a direct
pathogenic-signal in ALI [14]. It has been demonstrated that the
over-expression of these pro-inflammatory mediators contribute to ALI or lung
cancer [15]. Thus, down-regulation of TNF-α, IL-2, IL-6 and IL-8 expression
derived from 20E is beneficial in reducing the lung damage of mice. Similar
studies are also reported in ALI cases. Administration of
dexamethasone-21-acetate can dramatically reduce the concentration of cytokines
TNF-α, IL-2, IL-6 against the damage of LPS on lung [16]. In addition, 1α,
25-dihydroxyvitamin D3 and vitamin D3 analogue can attenuate the innate
immunity response in a mouse model of ALI through suppression of IL-8
production [17,18]. Notably, wide applications of steroids are observed in
medicine besides dexamethasone-21-acetate, 1α, 25-dihydroxyvitamin D3 and
vitamin D3 analogue [19-21]. Thus, these studies should open a new road to
explore of 20E functions.
Further research showed the expressions of IL-4 and
IL-10 were obviously induced in the middle-dose group of 20E and high-dose
group of 20E, suggesting multiple targets acted on by 20E are involved in the
protection event against ALI. Inflammatory response of organs is coordinated by
the interplay of anti-inflammatory cytokines and pro-inflammatory cytokines,
where a low concentration of pro-inflammation cytokines and/or a high
concentration of anti-inflammatory cytokines contribute to protection of
tissues and cells against damage and sustain homeostasis of body [22,23].
Meanwhile, the IL-4 and IL-10, as anti-inflammatory cytokines, their expression
levels that is induced by 20E t is helpful in inhibiting the expression of
pro-inflammatory cytokines [24,25]. For example, IL-10 works as a key player to
reduce the release of pro-inflammatory mediators during ALI, such as IL-6 and
inducible nitric oxide synthase [24,26]. Therefore, we postulate that the
relative level of anti-inflammatory cytokines and inflammatory cytokines play a
crucial role in organs against injury [22,23].
In the current study, protective-role of 20E against
ALI in the mice showed a dose-dependent effect suggesting that different
targets acted on by 20E are likely involved in inflammation of ALI. In the
insect and crustacean, 20E, as an important hormone that plays a key role in
regulating development, growth, reproduction and innate immunity by acting on
different targets [27,28]. Considering here, the study of TNF-α, IL-2, IL-6 and
IL-8, IL-4 and IL-10 temporal and spatial expressions derived from 20E
activation is an interesting work. Meanwhile, regulation of TNF-α, IL-2, IL-6
and IL-8, IL-4 and IL-10 derived from different-dose 20E treatment groups is
complex event. Therefore, great efforts are required to elucidate the
mechanisms of 20E in ALI.
ACKNOWLEDGEMENT
This research was funded by the National Natural
Science Foundation of Henan (No. 18A330004, PXY-BSQD-2018009, 17A180010,
PXY-PYJJ-2018005) and China Postdoctoral Science Foundation Funded Project
(2016M590143).
CONFLICT OF INTEREST
We declare that there is no
conflict of interest.
1. Nakagomi
T, Kitada O, Kuribayashi K, Yoshikawa H, Ozawa K, et al. (2004). The 150 kDa oxygen-regulated
protein ameliorates lipopolysaccharide-induced acute lung injury in mice. Am J
Pathol 165: 1279-1288.
2. Sachs
UJ (2011) Recent insights into the mechanism of transfusion-related acute lung
injury. Curr Opin Hematol 18: 436-442.
3. Ebihara
I, Hirayama K, Nagai M, Kakita T, Sakai K, et al. (2011) Angiopoietin balance
in septic shock patients with acute lung injury: Effect of direct hemoperfusion
with polymyxin B-immobilized fiber. Ther Apher Dial 15: 349-354.
4. Wilson
CN, Vance CO, Doyle TM, Brink DS, Matuschak GM, et al. (2012). A novel
post-exposure medical counter measure L-97-1 improves survival and acute lung
injury following intratracheal infection with Yersinia pestis. Innate Immun 18: 373-389.
5. Hu
LX, Du YY, Zhang Y, Pan YY (2012) Lack of association between interleukin-8-251
target; A polymorphism and colorectal cancer risk: A meta-analysis based on
3,019 cases and 3,984 controls. Asian Pac J Cancer Prev 13: 5075-5079.
6. Ju
ST, Sharma R, Gaskin F, Fu SM (2012) IL-2 controls trafficking receptor gene
expression and Th2 response for skin and lung inflammation. Clin Immunol 145:
82-88.
7. Zhang
CR, Lin JC, Xu WM, Li M, Ye HS, et al. (2013) Interleukin-12 and interleukin-2
alone or in combination against the infection in invasive pulmonary
aspergillosis mouse model. Mycoses 56: 117-122.
8. Hung
TJ, Chen WM, Liu SF, Liao TN, Lee TC (2012) 20-Hydroxyecdysone attenuates TGF-β
1-induced renal cellular fibrosis in proximal tubule cells. J Diabetes
Complicat 26: 463-469.
9. Jian
CX, Liu XF, Hu J, Li CJ, Zhang G, et al. (2013) 20-hydroxyecdysone-induced bone
morphogenetic protein-2-dependent osteogenic differentiation through the ERK
pathway in human periodontal ligament stem cells. Eur J Pharmacol 698: 48-56.
10. Kumpun S, Girault JP, Dinan L, Blais C, Maria
A, et al. (2011) The metabolism of 20-hydroxyecdysone in mice: Relevance to
pharmacological effects and gene switch applications of ecdysteroids. J Steroid
Biochem 126: 1-9.
11. Foucault AS, Mathé V, Lafont R, Even P, Dioh
W, et al. (2011) Quinoa extract enriched in 20-hydroxyecdysone protects mice
from diet-induced obesity and modulates adipokines expression. Obesity 20:
270-277.
12. Xia
XC, Yuan GQ, Zhang QY, Liu RZ, Wang ZX, et al. (2014) Effects of
20-hydroxyecdysone on improving memory deficits in streptozocin-induced type 1
diabetes mellitus in rat. Eur J Pharmacol 740: 45-52.
13. Sharma
R, Sung SS, Gaskin F, Fu SM, Ju ST (2012) A novel function of IL-2:
chemokine/chemoattractant/retention receptor genes induction in Th subsets for
skin and lung inflammation. J Autoimmun 38: 322-331.
14. Fang
Y, Xu P, Gu C, Wang Y, Fu XJ, et al. (2011) Ulinastatin improves pulmonary
function in severe burn-induced acute lung injury by attenuating inflammatory
response. J Trauma 71: 1297-1304.
15. Yao
HY, Zhang LH, Shen J, Shen HJ, Jia YL, et al. (2011) Cyptoporus polysaccharide
prevents lipopolysaccharide-induced acute lung injury associated with
down-regulating toll-like receptor 2 expression. J Ethnopharmacol 137:
1267-1274.
16. Yubero
S, Manso MA, Ramudo L, Vicente S, De Dios I (2012) Dexamethasone down-regulates
the inflammatory mediators but fails to reduce the tissue injury in the lung of
acute pancreatitis rat models. Pulm Pharmacol Ther 25: 319-324.
17. Takano Y, Mitsuhashi H, Ueno K (2011)
1α,25-di-hydroxyvitamin D₃ inhibits neutrophil recruitment in hamster model of
acute lung injury. Steroids 76: 1305-1309.
18. Takano
Y, Mitsuhashi H, Ishizuka S, Takahashi K, Chokki M, et al. (2012) TEI-A00114: A
new vitamin D3 analogue that inhibits neutrophil recruitment in an acute lung
injury hamster model while showing reduced hypercalcemic activity. Steroids 77:
1535-1542.
19. Jules-Elysee
KM, Wilfred SE, Memtsoudis SG, Kim DH, YaDeau JT, et al. (2012) Steroid
modulation of cytokine release and desmosine levels in bilateral total knee
replacement: A prospective, double-blind, randomized controlled trial. J Bone
Joint Surg Am 94: 2120-2127.
20. Wigenstam E, Jonasson S, Koch B, Bucht A
(2012) Corticosteroid treatment inhibits airway hyper responsiveness and lung
injury in a murine model of chemical-induced airway inflammation. Toxicology
301: 66-71.
21. Chazenbalk G, Singh P, Irge D, Shah A, Abbott
DH (2013) Androgens inhibit adipogenesis during human adipose stem cell
commitment to pre-adipocyte formation. Steroids 78: 920-926.
22. Sadr K, Walker RH (2012) The balancing act of
the inflammatory cascade after bilateral total knee arthroplasty commentary on
an article by Kethy M. Jules-Elysee, MD, et al.: ‘‘steroid modulation of
cytokine release and desmosine levels in bilateral total knee replacement. A
prospective, double-blind, randomized controlled trial’’. J Bone Joint Surg Am
94: e1791-1792.
23. Yang
R, Yang L, Shen X, Cheng W, Zhao B, et al. (2012) Suppression of NF-κB pathway
by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung
injury in mice. Eur J Pharmacol 674: 391-396.
24. Sun L, Cornell TT, LeVine A, Berlin AA,
Hinkovska-Galcheva V, et al. (2013) Dual role of interleukin-10 in the
regulation of respiratory syncitial virus (RSV)-induced lung inflammation. Clin
Exp Immunol 172: 263-279.
25. Wechsler
ME (2013) Inhibiting interleukin-4 and interleukin-13 in difficult-to-control
asthma. N Engl J Med 368: 2511-2513.
26. Ma HJ, Huang XL, Liu Y, Fan YM (2012) Sulfur
dioxide attenuates LPS-induced acute lung injury via enhancing
polymorphonuclear neutrophil apoptosis. Acta Pharmacol Sin 33: 983-990.
27. Flatt T, Heyland A, Rus F, Porpiglia E,
Sherlock C, et al. (2008) Hormonal regulation of the humoral innate immune
response in Drosophila melanogaster.
J Exp Biol 16: 2712-2724.
28. Wang
JL, Liu XS, Zhang Q, Zhao HB, Wang YF (2012) Expression profiles of six novel
C-type lectins in response to bacterial and 20E injection in the cotton
bollworm (Helicoverpa armigera). Dev
Comp Immunol 7: 221-232.