1347
Views & Citations347
Likes & Shares
Background: Immune-mediated hemolytic anemia as a result of intravenous (IV) contrast medium (CM) injection has been described 3 previous times in the literature and is thought to be mediated by drug-dependent autoantibodies. Checkpoint inhibitor therapies, such as nivolumab, and other investigational cancer treatments aim at increasing immune systemic activity against malignancy, however, they can lead to inappropriate immune activation, with off target immune effects.
Case Report: We observed a case of acute-onset severe hemolytic anemia which developed immediately after IV CM administration in a 56-year-old woman with non-small cell lung cancer (NSCLC) who was involved in clinical trial with investigational PEGylated recombinant human IL-10 (AM0010) and nivolumab. Work up was consistent with drug-induced immune hemolytic anemia, with evidence of anemia, elevated bilirubin, elevated LDH, and low haptoglobin, along with positive Direct Coombs test with anti-C3 direct antiglobulin test (DAT) and anti-IgG DAT. Patient’s hemolysis resolved rapidly with supportive care and steroids. We postulated a molecular mechanism through which the nivolumab and IL-10 administration may have pre-disposed to development of autoantibodies that led to hemolytic anemia in correlation with CM injection.
Results: Drug-induced immune hemolytic anemia resolved following treatment with steroids. Patient achieved a complete response while on clinical trial.
Conclusion: This is the fourth case published detailing immune-mediated hemolysis as a result of IV CM injection. Our case is unique given the fact that the patient was receiving immune-modulatory drugs while on clinical trial for NSCLC. We observed possible clinical correlation and postulated molecular mechanism to development of hemolytic anemia in correlation with CT contrast dye injection.
Keywords: IL-10, Nivolumab, Checkpoint Inhibitor, Hemolytic Anemia, DIIHA
ABBREVIATIONS
IV: Intravenous; CM: Contrast Medium; NSCLC: Non-small Cell Lung Cancer; LDH: Lactate dehydrogenase; DAT: Direct Antiglobin Testing; CT: Computed Tomography; Hb: Hemoglobin; PET-CT: Positron Emission Tomography-Computed Tomography; RUL: Right Upper Lobe; SUV: Standard Uptake Value; MRI: Magnetic Resonance Imaging; PRBC: Packed Red Blood Cell; CBC: Complete Blood Count; BMP: Basic Metabolic Panel; AST: Aspartate Transaminase; ALT: Alanine Transaminase; DIIHA: Drug Induced Hemolytic Anemia; RBC: Red Blood Cell; AIHA: Autoimmune Hemolytic Anemia
INTRODUCTION
This case report details a patient who developed severe hemolytic anemia following IV contrast medium (CM). There are a variety of iodinated compounds used as CM for diagnostic procedures, including imaging such as computed tomography (CT). These compounds include four subclasses consisting of nonionic, ionic, monomeric, or dimeric substances. The subclass of nonionic iodinated compounds includes iomeprol, iopamidol, iohexol and iotrolan. These compounds have been well-studied, and known side effects include localized pain, heat sensations, taste disturbances, and pseudo allergic reactions [1]. However, there have been two previously reported cases of immune-mediated hemolytic anemia following administration of iomeprol (Imeron) [2,3]. In addition, there has been one report of complement-mediated hemolysis following injection of an older ionic contrast medium, metrizoate (Isopaque) [4]. These cases occurred in female patients who had previously tolerated CM and included various markers of immunemediated hemolytic anemia. These patients also had symptoms of agitation, tachycardia, vomiting, decreases in hemoglobin (Hb) concentrations, increases in lactate dehydrogenase, positive C3d-DAT, and presence of CM-dependent antibodies [2,3]. Here we describe a subsequent unexplained case of immune-mediated hemolytic anemia following CT imaging with iohexol (Omniapaque) CM in a 58 years old female while on treatment for adenocarcinoma of the lung with nivolumab and IL-10 therapy.
CASE DESCRIPTION
The patient was entered on a clinical trial of combination therapy of AM0010, a long-acting PEGylated construct of recombinant human IL-10, and nivolumab, an anti-PD-1 checkpoint inhibitor, in May of 2018. A prior Phase 1/1b clinical trial of single agent AM0010 showed a good safety/tolerability prolife for AM0010 and demonstrated sustained anti-tumor effects with several cancers such as non-small cell lung cancer, advanced renal cell carcinoma and melanoma. The most common side effects reported in the clinical trial were fatigue, anemia, and thrombocytopenia [5]. However, in this patient, we observed a unique case of immune-mediated hemolytic anemia immediately after IV CM administration.
MATERIALS AND METHODS
The patient, a 56-year-old female (patient #01), was diagnosed in October 2015 with metastatic non-small cell lung carcinoma. Her primary tumor was an initial mass of 8 x 7 cm in the right upper lobe of the lung invading the mediastinum and also had a metastatic lesion on her right adrenal gland, consistent with a diagnosis of stage IV disease. She began treatment of paclitaxel/carboplatin chemotherapy for 4 cycles followed by radiation targeted to the lung and adrenal masses from the period of November 2015 to January 2016. In February 2018, a PET-CT scan identified a recurrent lesion in her RUL and an increasing standard uptake value (SUV) of 7.7 units, consistent with progressive disease. The patient began having severe headaches and expressive aphasia, in March of 2018. A brain MRI showed another recurrent mass in the left temporal lobe. Surgical resection identified metastatic non-small cell carcinoma; therefore, she received stereotactic radiation to her left temporal lobe bed. A PET-CT in April 2018 determined progression of disease in the RUL with a SUV of 11.2 units. At this time the patient decided to enroll in a clinical trial with combination therapy of nivolumab and AM0010.
The clinical trial began 6/27/2018 with dosing of nivolumab, 240 mg IV q 2 weeks, and AM0010, 0.8 mg subq qd. About a month after starting treatment, she developed severe anemia with hemoglobin decreased from 11.1 g/dL at baseline to 6.6, requiring packed red blood cell (PRBC) transfusion. The anemia was found to be associated with increased ferritin, negative Direct Coomb’s test, and normal bilirubin, lactate dehyodrogenase (LDH), and haptoglobin levels [6]. The anemia was attributed to IL-10 therapy and stabilized with dose reduction of the IL-10 from daily dosing to dosing 2 days/week. The follow-up Hb’s after the dose reduction were within normal range (9.4 to 11.1 g/dL).
She remained stable with the adjusted dosing of IL-10 twice a week from September 2018 to August 2019 with sustained treatment response. Her PET-CT scan on 9/4/2018, revealed a marked reduction in SUV of the RUL mass from 11.2 to 2.7, consistent with complete metabolic response to therapy. All other PET scans and brain MRIs during this time period also demonstrated sustained treatment response.
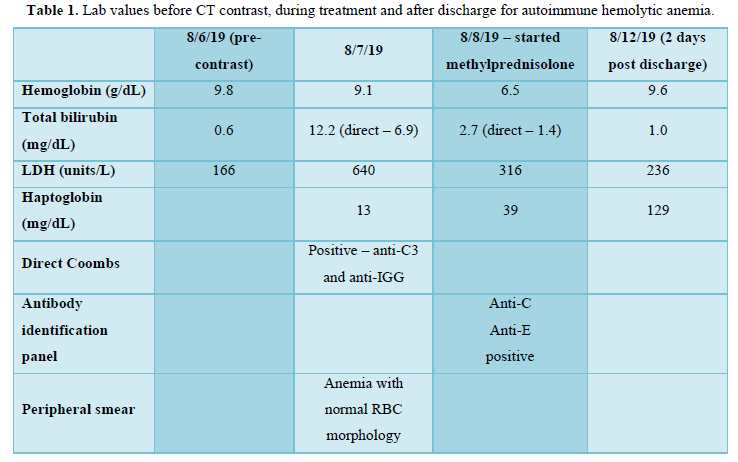
On August 6th, 2019, the patient underwent routine CT scan with contrast (Omnipaque 350), with acute development of severe hemolytic anemia. Labs drawn immediately prior to the CT-scan were normal (Hb of 9.8 g/dL, bilirubin of 0.6 mg/dL, and LDH of 166 units/L) (Table 1). However, within minutes of contrast exposure patient #01 developed severe bilateral back pain, headache, and an episode of vomiting. She was sent to the emergency room where she had an episode of diarrhea, but no significant change in CBC and BMP were observed, although bilirubin and liver enzymes were not drawn. She was briefly treated with IV morphine, ondansetron, and a normal saline fluid bolus then discharged. That night, the patient reported hematuria.
RESULTS
The patient presented to clinic the following morning and appeared severely jaundiced with evidence of scleral icterus and yellowing of the skin and soft palate. Repeat labs were performed which revealed Hb of 9.1 g/dL, total bilirubin of 12.2 mg/dL, direct bilirubin of 6.9 mg/dL, LDH of 640 units/L, reticulocyte of 2.99%, and an absolute reticulocyte count of 0.0957 10^6/uL (Table 1). She was admitted to the hospital for additional evaluation and management. She was found to have a haptoglobin of 13 mg/dL and a Direct Coombs test that was positive for both anti-C3 DAT and anti-IgG DAT (Table 1). Her peripheral smear showed normal RBC morphology. Her Hb dropped to 6.5 g/dL the second day of admission (Table 1), therefore she was given a transfusion of packed RBCs. Further work-up, showed a cold agglutination test with a negative result and antibody identification panel with anti-C and anti-E antibodies. An ultrasound of her liver showed normal echogenicity, no focal lesions, or dilation of the intrahepatic bile ducts. She had a fever, hypotension, and mildly elevated AST/ALT. There was a concern for disseminated intravascular coagulation as she had a d-dimer of 12.19 and FDP >20 ug/mL, however she did not develop any significant bleeding. Workup for infectious causes including blood cultures, HIV, CMV, and Hep B and C antibodies were all negative.
She was treated with steroids with dosing of methylprednisone 1 g/day for 3 days and her anemia improved to baseline and vitals stabilized. She was discharged home to complete prednisone taper from dosing of 1 mg/kg. Workup done a month later resulted in labs of: Hb 12.5 g/dL, bilirubin of 0.6 mg/dL, LDH of 180 units/L, and normalization of her AST/ALT. On 9/12/2019, a repeat PET scan exhibited no-hyper metabolic activity in the right upper lobe and no other areas concerning for disease, consistent with a complete response.
DISCUSSION
Immune-mediated hemolytic anemia following IV contrast injection has only been reported 3 times in the literature, with 2 cases being reported with iomeprol, and 1 case with metrizoate [2-4]. Both iomeprol and iohexol, the agent our patient received, are low-osmolar contrast mediums, as opposed to metrizoate, which is a high-osmolar contrast medium, which is no longer used due to increased rates of adverse events (AE’s) [7]. One of the cases with iomeprol demonstrated DAT only positive for anti-C3d antibodies [2], while in the other case the patient developed both anti-C3d and anti-IgG antibodies [3], similar to our patient.
Drug-induced immune hemolytic anemia (DIIHA) has been reported with a variety of drugs, most commonly cephalosporins. Two mechanisms of DIIHA have been proposed. The first involves drug-independent auto antibodies, which react to red blood cells (RBC’s) even without the presence of the drug and are thought to be mediated by cross-reactivity of drug antigen and red cell antigens, of which methyldopa is the most commonly implicated drug [8,9]. The second mechanism of DIIHA is the production of drug-dependent auto antibodies, which are thought to cause RBC hemolysis by binding to the drug that is bound to proteins on the RBC membrane, thereby causing RBC destruction via antibody-dependent cell-mediated cytotoxicity, with or without complement activation [8]. A few confounding factors in this unique case are the fact that the patient was receiving nivolumab in combination with pegylated IL-10. Since this case developed so acutely after receiving contrast medium, it seems most consistent with the second pattern of DIIHA, which is through the development of drug-dependent auto antibodies. Therefore, we do not think that the nivolumab and IL-10 were the causative agents, however, there is a question of whether these treatments pre-disposed to the development of drug-dependent immune-mediated hemolysis. Nivolumab has been associated with development of immune-mediated hemolytic anemia in multiple patients and has been associated with development of anti-IgG and anti-C3 antibodies [9].
As for pegylated IL-10, we have already shown a possible relationship between this therapy and non-immune-mediated anemia [6] however, it is yet to be determined if this therapy has a relation to autoantibody production as well. IL-10 is produced by a number of immune cells including macrophages, dendritic cells, B cells, and CD4+ T cells. CD4+ T cells are present in the tumor microenvironment and their production of IL-10 is mediated by multiple transcription factors including STATs, SMAD4, cJUN and GATA3 [10]. While IL-10 plays numerous roles, including Th2 immune regulation, another important role is the regulation of IL-12 production. IL-12 is a potent cytokine that activates the Th1 immune response that is tightly regulated, and the balance between IL10/IL-12 has been used as a biomarker in immune related diseases. Increased IL-10 levels and decreased IL-12 levels have been seen in patients with AIHA, and it is thought that this imbalance is involved in the production of auto antibodies leading to AIHA [11,12].
CONCLUSION
In this case, the positive anti-C3 and IgG DAT test, and acute onset immediately following contrast administration, make DIIHA from contrast administration most likely. Furthermore, we hypothesize, that prolonged immune stimulation with nivolumab, causes T cell proliferation which further increased the levels of IL-10 in the tumor microenvironment. This, in combination with the exogenous administration of IL-10 will require further investigation but may have further exacerbated the delicate balance of IL-10/IL-12 and contributed to the production of drug-dependent auto antibodies, which led to profound hemolysis in the presence of IV contrast medium. Given the rarity of contrast-induced hemolysis, this paper also highlights a rare but serious complication to keep in mind with patients who present with acute anemia after receiving CT contrast, especially if they are undergoing treatment with IL-10 and/or checkpoint inhibitor.
- Dooley M, Jarvis B. (2000) Iomeprol: A review of its use as a contrast medium. Drugs 59: 1169-1186.
- Mayer B, Leo A, Herziger A, Houben P, Schemmer P, et al. (2013) Intravascular immune hemolysis caused by the contrast medium iomeprol. Transfusion 53: 2141-2144.
- Maurin C, Vassal O, Darien M, Raba M, Allaouchiche B, et al. (2018) Immune hemolysis secondary to injection of contrast medium. Transfusion 58: 2113-2114.
- Nordhagen R, Vik H, Wolthuis K, Bohn HP, Urdahl P, et al. (1991) Immune-mediated hemolysis associated with the administration of a radiographic contrast medium. Transfusion 31: 843-846.
- Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, et al. (2016) Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. J Clin Oncol 34: 3562-3569.
- Choucair K, Kelso JD, Duff JR, Cassidy CS, Albrethsen MT, et al. (2019) Interleukin 10-Mediated Response and Correlated Anemia in a Patient with Advanced Non-Small Cell Lung Carcinoma. Case Rep Oncol 12: 297-303.
- Seong JM, Choi NK, Lee J, Chang Y, Kim YJ, et al. (2013) Comparison of the safety of seven iodinated contrast media. J Korean Med Sci 28: 1703-1710.
- Garratty G, Petz LD (1975) Drug-induced immune hemolytic anemia. Am J Med 58: 398-407.
- Tanios GE, Doley PB, Munker R (2019) Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol 102: 157-162.
- Saraiva M, O'Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170-181.
- Fagiolo E, Toriani-Terenzi C (2002) Th1 and Th2 Cytokine Modulation by IL-10/IL-12 Imbalance in Autoimmune Haemolytic Anaemia (AIHA). Autoimmunity 35: 39-44.
- Toriani-Terenzi C, Fagiolo E (2005) IL-10 and the cytokine network in the pathogenesis of human autoimmune hemolytic anemia. Ann N Y Acad Sci 1051: 29-44.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)

