Review Article
Isolation and Screening of Laccase Producing Fungi from Different Regions of Western Ghats
1421
Views & Citations421
Likes & Shares
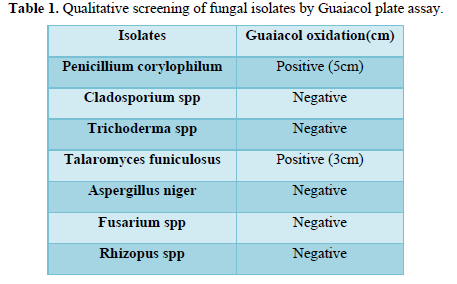
In forest ecosystem activities of ligninolytic enzymes (laccase, Mn- peroxidase and lignin-peroxidase) produced by microbes play a vital role, through the breakdown of plant cell wall polymers, including the recalcitrant lignin. The present study aims at isolation and screening of laccase producing fungi from forest litter. In this study seven fungal isolates were isolated on Potato Dextrose Agar (PDA) plates using serial dilution technique. The two elite fungal isolates Talaromyces funiculosus (3cm) and Penicillium corylophilum (5cm) showed good laccase activity on guaiacol plates. From quantitative laccase-enzyme assay the elite fungal isolates such as Talaromyces funiculosus and Penicillium corylophilum showed the laccase activity of 0.00096 U/ml and 0.00033 U/ml respectively.
Keywords: Laccase, Guaiacol Indicator, Qualitative, Quantitative, Fungi, A1 (Talaromyces funiculosus), A2 (Penicillium corylophilum)
INTRODUCTION
Laccase (p-diphenol: EC 1.10.3.2 oxygenic reductase) degrades several phenolic compounds [1]. Laccase is also known as multi-copper blue oxidizes, it belongs to oxidoreductase group of enzyme. 50 kDa and 130 kDa are their biochemically glycoprotein carrying molecular mass [2]. A fungus produces more than one isoform of laccase [3]. Fungal laccase is multinuclear, extracellular, mostly inducible, monomeric glycoproteins with 10-20% of carbohydrate contents. It makes laccase to high stable [4]. Fungal laccases play a greater role in fungal pathogen host interactions, stress preservation, morphogenesis and lignin degradation [5]. Fungi belonging to deuteromycetes, basidiomycetes and ascomycetes are known to biotechnologically important and environmentally significant lacquer users, such as in bioremediation and biodegradation processes [3, 4, 6, 7]. Laccase are used as new biocatalysts for organic synthesis [4, 8]. They are used in beverage or food to improve their appearance. It removes the harmful phenolic compounds responsible for turbidity and browning in milk, wine and fruit juice [9]. Laccase is capable to depolymerize and de-lignify wood pulp fiber and is used in chlorine-free in bio-palpation process [10]. Their potential application in textile and dye industries for the enzymatic modification of bleaching dye [10, 11]. Laccase degrade recalcitrant and xenobiotic compounds; it is a major contamination source in soil [12]. Laccase also degrade PHAs, which shows mutagenic, carcinogenic and cytotoxic properties that responsible for risk to human health [13]. In the present study Forest soil samples were subjected to serial dilution on PDA. Isolates from that were subjected on PDA containing Guaiacol for their qualitative laccase screening. The elite isolates from this qualitative screening were then further subjected for quantitative estimation of laccase.
MATERIALS & METHODS
Materials required
Potato Dextrose Agar, Potato Dextrose Broth, Guaiacol, Sodium Acetate Buffer and Tetracycline were procured from Hi-Media, Mumbai (India).
Collection of soil sample and isolation of fungi
Samples of forest litter were collected from Rippon pet and Kodachadri in Hosanagara Taluk, Shimoga district in a sterile plastic bag and were subjected to serial dilution for isolation of the fungi. 1 g of sample soil was blended into 10 ml of sterile water. The suspension was serially diluted, diluted samples were spread plated on Potato Dextrose Agar medium. Plates were incubated at 28°C for 7 days [14].
Qualitative screening
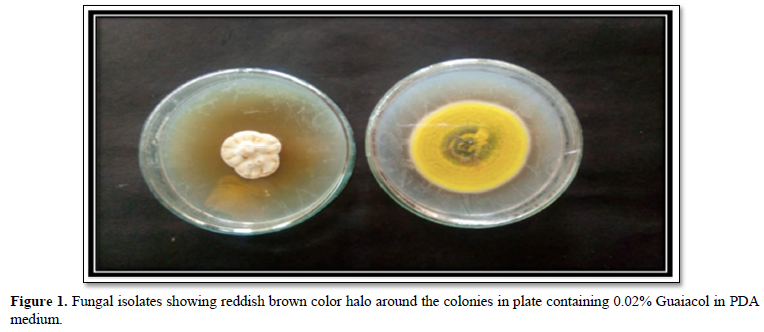
The isolated fungal cultures were further subjected to qualitative screening on Potato Dextrose Agar plates containing 0.02% Guaiacol. The plates were incubated for 5 days at 30°C. The laccase producing fungal strains showed reddish brown halo around the colonies in the plates containing Guaiacol, after 5 days of incubation. The elite isolates from qualitative screening were further subjected to quantitative estimation of laccase [15].
Quantitative screening
The two positive elite fungal isolates from qualitative screening were inoculated into Erlenmeyer flask (250ml), containing 100ml Potato Dextrose Broth, for quantitative screening were incubated at room temperature for 15 days (Table 1 and Figures 1 and 2).




Extracellular enzyme activity
- After incubation crude extract were filtered using Whatman no 1. At room temperature, 10mM of Guaiacol and 100mM of sodium acetate buffer (pH 5.0) were used to test the laccase activities. The reaction mixture includes 1ml of Guaiacol, a buffer of 3ml sodium acetate and a source of 1ml of enzyme. The reaction was incubated for 10 minutes at 30°C. Laccase activity is calculated in U / ml which defines the enzyme output quantity for one micromole colored product per ml per minute. Reaction mixture absorbance was measured at 470 nm using UV Spectrophotometer [15].
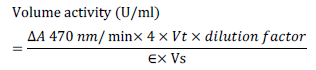
Calculation where,
Vt = final volume of reaction mixture (ml) = 5.0, Vs = sample volume (ml) = 1, € = extinction co-efficient of Guaiacol = 6,740/M/cm, 4 = derived from unit definition & principle
Molecular characterization of fungal isolates
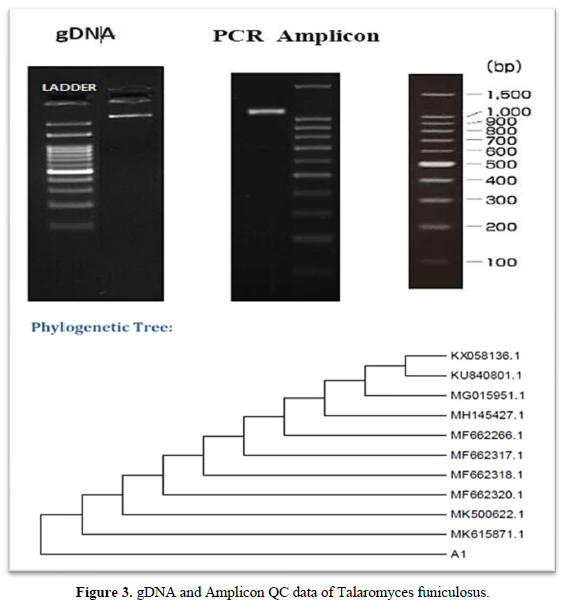
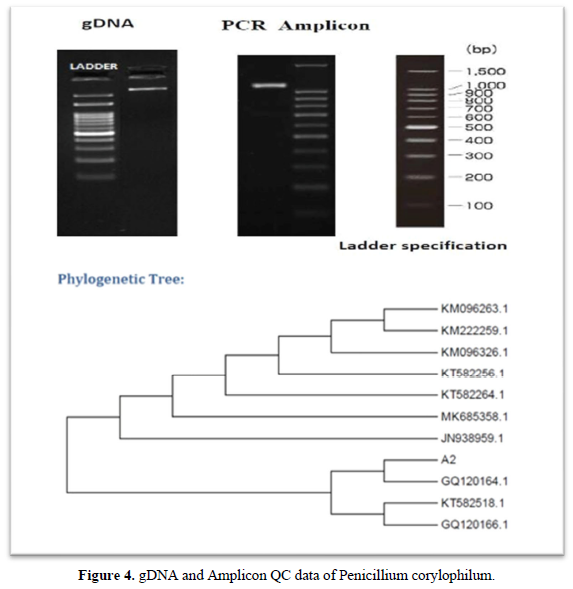
- DNA was isolated from the culture. Its quality was evaluated on 1.0 % agarose gel, a single band of high-molecular weight DNA has been observed.
- Fragment of 18S rRNA gene was amplified by NS1 and NS4 primers. A single discrete PCR amplicon band of 1100 bp was observed when resolved on agarose gel.
- The PCR amplicon was purified to remove contaminants.
- Forward and reverse DNA sequencing reaction of PCR amplicon was carried out with NS1 and NS4 primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer.
- Consensus sequence of 18S rRNA gene was generated from forward and reverse sequence data using aligner software.
The 18S rRNA gene sequence was used to carry out BLAST with the ‘nr’ database of NCBI GenBank database. Based on maximum identity score first ten sequences were selected and aligned using multiple alignment software program Clustal W. Distance matrix was generated using RDP database and the phylogenetic tree was constructed using MEGA 6 [16, 17].
RESULTS
The present study focused primarily on isolating and screening the laccase producing fungi from forest litter of Western Ghats (South India). Eleven fungal isolates were isolated from soil samples. The Potato Dextrose Agar was used for maintenance of fungal isolates. They were qualitatively screened with Guaiacol as a substrate for their future production capacity of laccase. The potato dextrose agar plates which contain 0.02% Guaiacol were inoculated with all fungal isolates and incubated at 30°C for 5 days. Table 1, shows the results of screening tests. The fungal isolates developed reddish brown halo around the colonies after 5 days of incubation, indicates positive for laccase production and lack of reddish brown halo indicates negative for laccase production. The two elite fungal isolates Talaromyces funiculosus (3cm) and Penicillium corylophilum (5cm) showed good laccase activity on Guaiacol plate test. Similar work carried out by [18] isolated laccase producing fungi on PDA plates containing 4 mM of guaiacol showed reddish brown oxidation zone. Rehan et al. [18] also screened laccase producers such as Trichoderma harzianum on potato dextrose agar supplemented with 0.04% guaiacol. The Talaromyces funiculosus and Penicillium corylophilum elite fungal isolates showed the maximum laccases production of 0.00096 U/ml and 0.00033 U/ml in Potato dextrose bouillon following 15 days of incubation at room temperature. Figure 3 and Figure 4 describes the molecular characterization of elite fungal isolates [19]. Senthivelan et al. [18] reported the laccase activity of white rot fungus 3.2 U/ml was measured by UV-Visible spectrophotometer using guaiacol as a substrate. Rehan et al. [18] reported the laccase production of 1.479 U/ml by Trichoderma harzianum which was measured by UV-Visible spectrophotometer using guaiacol as a substrate.


CONCLUSION
The two elite fungal isolates Talaromyces funiculosus and Penicillium corylophilum were good laccase producers from above screening process can be further used in development of potential consortium for composting of plant wastes.
ACKNOWLEDGEMENT
The authors are thankful to Department of Microbiology, Indian Academy Degree College (Autonomous), Bengaluru for providing the necessary research facilities and also thankful to colleagues for their guidance and support.
- Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273: 2308-2326.
- Morozova O, Shumakovich GP, Gorbacheva M, Shleev SV, Yaropolov AI (2007) “Blue” laccases. Biochem 72: 1136-1150.
- Hoshida H, Nakao M, Kanazawa H, Kubo K, Hakukawa T (2001) Isolation of five laccase gene sequences from the white-rot fungus Trametes sanguinea by PCR, and cloning, characterization and expression of the laccase cDNA in yeasts. J Biosci Bioeng 92: 372-80.
- Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60: 551-565.
- Thurston CF (1994) The structure and function of fungal laccases. Microbiol 140: 19-26.
- Desai SS, Nityanand C (2011) Microbial laccases and their applications: a review. Asian J Biotechnol 3: 98-124.
- Shraddha, Shekher R, Sehgal S, Kamthania M, Kumar A (2011) Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme Res 2011: 21786.
- Mai C, Milstein O, Huttermann (1999) Fungal laccase grafts acrylamide onto lignin in presence of peroxides. Appl Microbiol Biot 51: 527-31.
- Couto SR, Herrera JL (2006) Industrial and biotechnological applications of laccases: A review. Biotechnology advances 24: 500-513.
- Kunamneni A, Ghazi I, Camarero S, Ballesteros A, Plou FJ et al. (2008) Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem 43: 169-178.
- Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A et al. (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66: 3357-3362.
- Osma JF, Toca-Herrera JL, Rodrıguez-Couto S (2006) Laccase production by Trametes pubescens grown on wheat bran under solid-state conditions.
- Bamforth SM, Singleton I (2005) Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80: 723-736.
- Waksman SA (1992) A method for counting the number of fungi in the soil. J Bacteriol 7: 339-341.
- Vadapally P, Gudikandula K, Maringanti SC (2015) Isolation, Screening and Identification of Laccase Producing Fungi from Eturnagaram Forest, Warangal District, Telangana, India. Sci Technol Arts Res J 4: 120-123.
- Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512-526.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725-2729.
- Vikineswary S, Abdullah N, Renuvathani M, Sekaran M, Pandey A et al. (2006) Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour Technol 97: 171-177.
- Nayak B, Choudhary R, Roymom M (2017) Lignocellulolytic fungal isolation and screening for their laccase producing ability. Indian J Sci Res 13: 188-191.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Astronomy and Space Research
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)





