196
Views & Citations10
Likes & Shares
α‐synucleinopathic cholinergic impairment including PD motor symptoms, gait dysfunction, levodopa-induced dyskinesias, cognitive deterioration, psychosis, sleep abnormalities, autonomic dysfunction, and altered olfactory function has long been treated using acetylcholinesterase inhibitors (AChEIs) [16-21]. These symptoms result from reduction of cholinergic tone in the striatum and/or degeneration of cholinergic nuclei, most importantly the nucleus basalis magnocellularis and the pedunculopontine nucleus [20].
A specific, reversible acetylcholinesterase inhibitor, Donepezil limits the action of the acetylcholine-hydrolyzing enzyme acetylcholinesterase, increasing acetylcholine levels which in turn can mitigate symptoms of cholinergic impairment [22-24]. Donepezil performs “dual action” in reducing cholinergic impairment, also independently facilitating neuronal nicotinic acetylcholine receptors [25], historically making it a drug of choice for managing symptoms of cholinergic impairment [21,22,25-27]. In PD and NCDLB patients, Donepezil does not exacerbate Parkinsonian symptoms or generate new symptoms, comparing favorably with other AChEIs [28-31]. In a study with a nongeriatric affective patient population with severe bowel immotility, the use of Donepezil reduced increased bowel contractions 477% [32,33].
Based on the body of research evidence cited above, it was hypothesized that in patients with Lewy body diseases including PD and NCDLB, Donepezil might reduce symptoms of constipation, obstipation and/or impaction thought to be manifestations of α‐synucleinopathy-based cholinergic impairment in the in the ENS, specifically the MP and CSMP. A secondary hypothesis posited that Donepezil might counteract bowel immotility associated with the use of Carbidopa-Levodopa.
CASE PRESENTATION
Donepezil was orally administered in daily doses varying from 5 to 10 mg to each of four PD and NCDLB patients (patients A, B, C and D) diagnosed at varying levels of disease progression with symptoms of constipation, obstipation and/or impaction to see whether Donepezil reduced bowel immotility symptoms including constipation, obstipation, and impaction [34]. In this context, “constipation” is defined as bowel movements less than once in two days, “obstipation” as less than once a week, and “impaction” as blockage of the lower intestine or complete cessation of bowel function [35].
PD was diagnosed for Patient A (male age 51, Donepezil initiated at age 53) and Patient B (male age 70, Donepezil initiated age 70) based on data from MRI’s, neurological assessments, CT scans and Modified Hoehn and Yahr (H & Y) scores [36]. NCDLB was diagnosed for Patient C (female age 69, Donepezil initiated age 69) and Patient D (male, age 74, Donepezil initiated age 78) based on data from MRI’s, CT scans, neurological evaluations, and scores on the Mini-Mental State Examination (MMSE), the Quick Dementia Rating System (QDRS) and the Lewy Body Composite Risk Score [37-39]. Patient B was also diagnosed with NCDLB six months after the initiation of Donepezil. Symptoms of constipation, obstipation and impaction were measured for each patient prior to and after treatment with Donepezil at intervals of two, four, and six weeks, and six, twelve, eighteen, thirty-six, forty-eight, sixty and seventy-two months.
RESULTS
In each of the four patients, assessment two weeks after initial oral administration of Donepezil at daily doses of 5 mg indicated significant reduction in the symptoms of constipation, obstipation and impaction. Increases in symptom reduction were observed at four weeks and six weeks. At each of these intervals, there was no exacerbation of existing symptoms, nor emergence of new symptoms [34], as shown in Table 1.
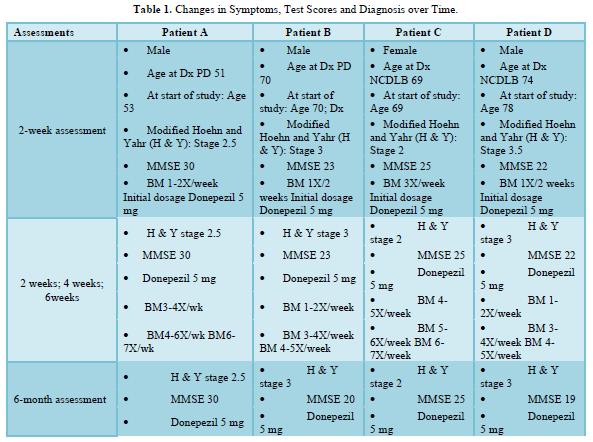
At six months, Patients A and C demonstrated no change in symptoms. For Patient B, self-report and test scores indicating cognitive changes led to the diagnosis of NCDLB, and increased dosage of Donepezil to 10 mg. Testing indicated α‐synuclein disease progression in cognition and movement for both Patients B and D without increased bowel immotility or emergence of new symptoms [40].
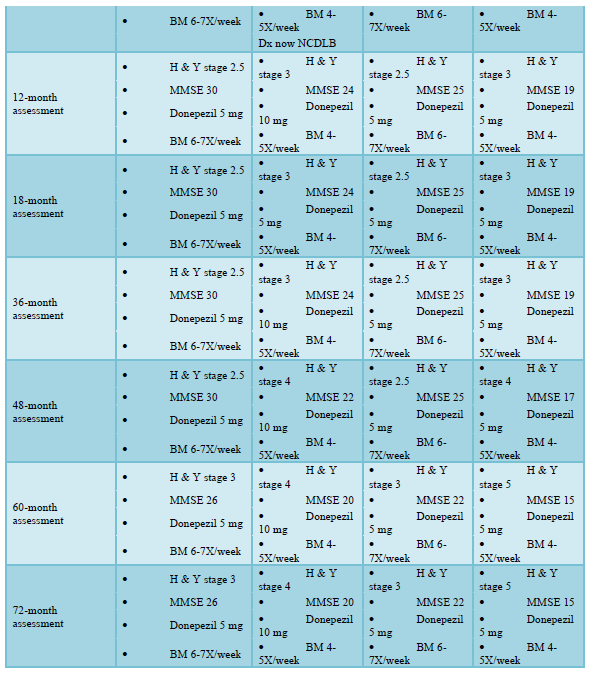
Assessment at twelve months showed no symptom exacerbation, except in Patient C, whose H & Y score indicated advancement of Parkinsonian symptoms [41]. Testing demonstrated recovery of some cognitive function (short-term memory and word-finding) in Patient B, whose dosage of Donepezil had been doubled at six months. At eighteen and twenty-four months, testing indicated no symptom change in any of the four patients [42].
Patient B had been using Levodopa-Carbidopa (Sinemet) for Parkinsonian features and Bupropion for depression. At twenty-four months, Patient B was prescribed Vortioxetine for its reported efficacy in reducing anxiety, depression, and cognitive impairment [43-64]. Within two weeks, he reported exacerbation of cognitive impairment and was hospitalized with bowel impaction. Less than 48 hours after discontinuing Vortioxetine, his bowel function and cognition returned to their pre-Vortioxetine levels [65].
At forty-eight months, Patients B and D showed progression of α‐synucleinopathy manifesting as declining MMSE scores and increasing movement disorders with higher H & Y scores [66]. At sixty months, MMSE scores indicated cognitive change in all four patients, and advancement of Parkinsonian movement symptoms in Patients A, C and D (with higher H & Y scores). However, even at seventy-two months there was no increase in bowel immotility for any of the patients. A summary of findings is shown in Table 1.
DISCUSSION AND CONCLUSION
Case study findings support the hypothesis that Donepezil can reduce symptoms of constipation, obstipation and/or impaction thought to be manifestations of α‐synucleinopathy-based cholinergic impairment in the in the ENS, specifically the MP and CSMP in patients with Lewy body diseases including PD and NCDLB, well as the secondary hypothesis that Donepezil can counteract bowel immotility associated with the use of Carbidopa-Levodopa.
A bonus finding was that Vortioxetine can inhibit serotonergic and cholinergic transmission, and importantly, that such inhibition is reversible [64]. Vortioxetine is metabolized by cytochrome P450 enzymes (e.g., CYP450 2D6) and subsequently by uridine diphosphate glucuronosyltransferase, and was initially thought to have relatively low risk for pharmacodynamic drug interactions [68-70]. The current case study demonstrated that significant increases in Vortioxetine peak plasma concentration and systemic exposure can occur when Vortioxetine is co-administered with the potent CYP450 2D6 inhibitor Bupropion [67], as well as Levodopa-Carbidopa (Sinemet) [71,72].
The molecular mechanisms underlying Vortioxetine’s site binding appear to utilize the same 5-HT3 receptor binding site used by serotonin selective reuptake inhibitors (SSRIs). Varying from currently known 5-HT3Aorthosteric ligands, Vortioxetine binds through interaction with residues of the aromatic box motif in the orthosteric binding site in a manner similar to the setron class of competitive antagonists and 5-HT. Vortioxetine also interacts with residues not previously considered relevant for the binding of either setrons or 5-HT, including Thr176 on loop B and Val202 on loop F [71]. Vortioxetine’s partial agonist activity can induce persistent and insurmountable inhibition, so that its peak plasma concentration and systemic exposure can more than double through its combined interactions with Bupropion and Levodopa-Carbidopa, significantly increasing its serotonergic inhibitory potential [68-72].
In the current case study, the combination of Vortioxetine, Bupropion and Levodopa-Carbidopa appears to have significantly increased Lewy Body α‐synuclein cholinergic suppression in Patient B’s ENS, rapidly inducing bowel immotility. It also produced significant cognitive interference due to the apparent combination of Vortioxetine’s cholinergic and serotonergic inhibition. These unanticipated findings contribute to a growing understanding of potential drug interactions for Vortioxetine [65,67].
However, the case study’s primary focus continues to be the efficacy of Donepezil, whose “dual action” independently facilitates neuronal nicotinic acetylcholine receptors, while specifically and reversibly limiting the action of the acetylcholine-hydrolyzing enzyme acetylcholinesterase [22-25]. The apparent elevation of acetylcholine levels through the combination of these two mechanisms appears to significantly mitigate symptoms attributable to cholinergic impairments, which include bowel slowing and cognitive interference [24].
The findings of this longitudinal case study are consistent with previous research indicating that Donepezil slows or reverses cognitive symptom progression in α‐synucleinopathy, including short-term memory loss, difficulty with word-finding, hallucinations and cognitive interference [16-20]. Moreover, although it appears that with advancing age and over a longer time frame α‐synucleinopathy eventually erodes cognitive and motor function, the current study’s findings suggest that the oral administration of Donepezil is a viable treatment protocol for mitigating α‐synucleinopathy-based ENS suppression of the cholinergic pathways in the MP and the CSMP, providing reduction in the symptoms of constipation, obstipation, and impaction. Future research is recommended over an extended time frame using larger numbers of subjects matched for diagnosis, age, gender and other variables.
AUTHOR CONTRIBUTION
The author warrants that he has reviewed and approved the manuscript prior to its submission, and assumes responsibility for the contents of the manuscript.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING
The author declares no conflicts of interest in the manuscript, including financial, consultant, institutional, and other relationships that might lead to bias or a conflict of interest. The author also declares no sources of funding for the manuscript. The Santa Barbara Cottage Hospital Institutional Review Board granted a waiver (#18-81ix) for this case study.
- Lebouvier T, Neunlist M, Bruley desVarannes S, Coron E, Drouard A, et al. (2010) Colonic Biopsies to Assess the Neuropathology of Parkinson’s Disease and Its Relationship with Symptoms. PLoS One 5(9): e12728.
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1989) Lewy Bodies in the enteric nervous system in Parkinson’s disease. Arch Histol Cytol 52(Supplement P): 191-194.
- Braaka H, de Vosb RAI, Bohlc J, Tredici KD (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 396(1): 67-72.
- Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33(6): 599-614.
- Castellanos AM, Chamorro CE, Sevilla FE, Moreno AO, Rebollo AC, et al. (2007) Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders? Neurology 68(23): 2012-2018.
- Holmqvist S, Chutna O, Bousset L, Kirk PA, Li W, et al. (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neurol Scand 128(6): 805-820.
- Iranzo A, Arcos AF, Tolosa E, Serradell M, Molinuevo JL, et al. (2014) Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 147 patients. PLoS One 9(2): e89741.
- Gjerløff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, et al. (2015) Imaging acetylcholinesterase density in peripheral organs in Parkinson's disease with 11C-donepezil PET. Brain 138(3): 653-663.
- Porter AJ, Wattchow DA, Brookes SJH, Costa M (2002) Cholinergic and nitrergic interneurons in the myenteric plexus of the human colon. Gut 51(1): 70-75.
- Molloy S, McKeith I, O’Brien J, Burn D (2005) The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 76(9): 200-1203.
- Dupont Pharmaceuticals: Product Information: Sinemet CR (carbidopa-levodopa), Wilmington, DE (2016).
- Jost WH, Schimrigk K (1991) Constipation in Parkinson’s disease. Klinische Wochenschrift 69(20): 906-909.
- Kaye J, Gage H, Kimber A, Storey L, Trend P (2006) Excess burden of constipation in Parkinson's disease: A pilot study. Mov Disord 21(8): 1270-1273.
- Phillips C, Polakoff D, Maue SK, Mauch R (2001) Assessment of Constipation Management in Long-Term Care Patients. J Am Med Dir Assoc 2(4): 149-154.
- Lepkowsky CM (2017) Donepezil for constipation in Lewy Body Diseases: Four case studies. Act Nerv Super 59(1): 19-27.
- Kosaka K, Oyanagi S, Matsushita M, Hori A (1976) Presenile dementia with Alzheimer-, Pick and Lewy-body changes. Acta Neuropathol 36(3): 221-233.
- Perry EK, Smith CJ, Court JA, Perry RH (1990) Cholinergic nicotinic and muscarinic receptors in dementia of Alzheimer, Parkinson and Lewy body types. J Neural 2(3): 149-158.
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47(5): 1113-1124.
- McKeith IG (2000) Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol Clin. 18: 865-902.
- Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Parkinson’s Disease 2: 16001.
- Lepkowsky CM (2017) Mechanisms of α‐synuclein pathology and treatment in the enteric nervous system. International J Genetic Sci 3(1): 1-6.
- Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, et al. (2001) Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer’s disease. Neurosci Lett 300(3): 157-160.
- Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J (2004) Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: The relationship between pharmacological effects and clinical efficacy. Drugs Aging 21(7): 453-478.
- Parsons CG, Danysz W, Dekundy A, Pulte I (2013) Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res 24(3): 358-369.
- Di Angelantonio S, Bernardi G, Mercuri NB (2004) Donepezil modulates nicotinic receptors of substantia nigra dopaminergic neurons. Br J Pharmacol 141(4): 644-652.
- Bosboom JLW, Stoffers D, Wolters EC (2004) Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm 111(10): 1303-1315.
- Minett TSC, Thomas A, Wilkinson LM, Daniel SL, Sanders J, et al. (2003) What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson's disease with dementia. Int J Geriatr Psychiatry 18(11): 988-993.
- Birks JS (2006) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Systematic Reviews: Dementia and Cognitive Improvement Group 2006(1): CD005593.
- Mori E, Ikeda M, Kosaka K (2012) Donepezil for dementia with Lewy bodies: a randomized, placebo controlled trial. Ann Neurol 72(1): 41-52.
- Mori E, Ikeda M, Nagai R, Matsuo K, Nakagawa M, et al. (2015) Long-term donepezil use for dementia with Lewy bodies: Results from an open-label extension of Phase III trial. Alzheimers Res Ther 7(1): 5.
- Rolinski M, Fox C, Maidment I, McShane R (2012) Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Systematic Reviews: Dementia and Cognitive Improvement Group. (2012) (3): CD006504.
- Jacobsen FM, Comas-Díaz L (1999) Donepezil for psychotropic-induced memory loss. J Clin Psychiatr 60(10): 698-704.
- Broad J, Kung VWS, Boundouki G, Aziz Q, De Maeyer JH, et al. (2013) Cholinergic interactions between donepezil and prucalopride in human colon: potential to treat severe intestinal dysmotility. Br J Pharmacol 170(6): 1253-1261.
- Lepkowsky CM (2016) Medications Linked to Cognitive Impairment in Older Adults. Pract Innov 1(4): 253-264.
- Lepkowsky CM (2022) Donepezil and α‐synuclein Constipation: A 60 Month Follow-Up. POJ Clin Case Rep 4(1): 1-8.
- Zhao YJ, Wee HL, Chan Y-H, Seah SH, Lok W, et al. (2010) Progression of Parkinson's disease as evaluated by Hoehn and Yahr stage transition times. J Mov Disord 25(6): 710-716.
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3): 189-198.
- Galvin J (2015) The Quick Dementia Rating System (QDRS): A rapid dementia staging tool. Alzheimers Dement (Amst) 1(2): 249-259.
- Galvin J (2015) Improving the clinical detection of Lewy body dementia with the Lewy body composite risk score. Alzheimers Dement (Amst) 1(3): 316-324.
- Lepkowsky CM (2017) Donepezil for Lewy Body constipation: A six-month follow-up. J Mol Genet Med 11: 3
- Lepkowsky CM (2018) Donepezil for Constipation in Lewy body disease: A twelve-month follow-up. J Mol Genet Med 12: 1
- Lepkowsky CM (2018) Donepezil for α‐synuclein Constipation: An 18 Month Follow-Up. POJ Clin Case Rep 1(1): 1-4.
- Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Reperant C, et al. (2013) Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioral and neurogenesis outcomes in mice. Neuropharmacology 73: 147-159.
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15(5): 589-600.
- Boulenger JP, Loft H, Florea I (2012) A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol 26(11): 1408-1416.
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME (2012) A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatr 73(7): 953-959.
- Jain R, Mahableshwarkar AR, Jacobsen PL, Chen Y, Thase ME (2013) A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol 16(2): 313-321.
- Mahableshwarkar AR, Jacobsen PL, Chen Y (2013) A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin 29(3): 217-226.
- Theunissen EL, Street D, Højer AM, Vermeeren A, van Oers A, et al. (2013) A randomized trial on the acute and steady-state effects of a new antidepressant, vortioxetine (Lu AA21004), on actual driving and cognition. Clin Pharmacol Ther 93(6): 493-501.
- Citrome L (2014) Vortioxetine for major depressive disorder: A systematic review of the efficacy and safety profile for this newly approved antidepressant-what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract 68(1): 60-82.
- Garnock-Jones KP (2014) Vortioxetine: a review of its use in major depressive disorder. CNS Drugs 28(9): 855-874.
- Connolly KR, Thase ME (2015) Vortioxetine: A New Treatment for Major Depressive Disorder. Expert Opin Pharmacother 17(3): 421-431.
- Jacobsen P, Mahableshwarkar AR, Serenko M, Chen Y, Trivedi M (2015) A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatr 76(5): 583-591.
- Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH (2015) A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology 232(12): 2061-2070.
- Mahableshwarkar AR, Jacobsen PL, Serenko M (2015) A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. J Clin Psychiatr 76(5): 583-591.
- Thase ME, Mahableshwarkar A, Dragheim M, Loft H, Vieta E (2016) A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol 26(6): 979-993.
- Mahableshwarkar AR, Jacobsen PL, Serenko M (2014a) A randomized, double-blind, fixed-dose study comparing the efficacy and tolerability of vortioxetine 2.5 and 10 mg in acute treatment of adults with generalized anxiety disorder. Hum Psychopharmacol 29(1): 64-72.
- Mahableshwarkar AR, Jacobsen PL, Chen Y, Simon JS (2014b) A randomized, double-blind, placebo-controlled, duloxetine-referenced study of the efficacy and tolerability of vortioxetine in the acute treatment of adults with generalised anxiety disorder. Int J Clin Pract 68(1): 49-59.
- Jensen JB, du Jardin KG, Song D, Budac D, Smagin G, et al. (2014) Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: evidence for direct 5-HT receptor modulation. Eur Neuropsychopharmacol 24(1): 148-159.
- McIntyre RS, Lophaven S, Olsen K (2014) A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharm 17(10): 1557-1567.
- Mahableshwarkar A, Zajecka J, Jacobson W, Chen Y, Keefe RS (2015) A Randomized, Placebo-Controlled, Active-Reference, Double-Blind, Flexible-Dose Study of the Efficacy of Vortioxetine on Cognitive Function in Major Depressive Disorder. Neuropsycho pharmacol 40(8): 2025-2037.
- Vieta W, Sluth LB, Olsen CK (2018) The effects of vortioxetine on cognitive dysfunction in patients with inadequate response to current antidepressants in major depressive disorder: A short-term, randomized, double-blind, exploratory study versus escitalopram. J Affect Disord 227: 803-809.
- Al-Sukhni M, Maruschak NA, McIntyre RS (2015) Vortioxetine: A review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin Drug Saf 14(8): 1291-1304.
- Lundbeck A/S. FDA updates Trintellix® (vortioxetine) label to include data showing improvement in processing speed, an important aspect of cognitive function in acute Major Depressive Disorder (MDD) (2018). Accessed on: September 18, 2018. Available online at: http://investor.lundbeck.com/news-releases/news-release-details/fda-updates-trintellixr-vortioxetine-label-include-data-showing
- Lepkowsky CM (2019) Vortioxetine and Lewy Body Disorders. Int Res J Pharm Med Sci 2(2): 2-20.
- Lepkowsky CM (2021) Donepezil and α‐synuclein Constipation: A 48 Month Follow-Up. POJ Clin Case Rep 3(1): 1-7.
- Lepkowsky CM (2019) Donepezil and α‐synuclein Constipation: A 36 Month Follow-Up. POJ Clin Case Rep 2(1): 1-7.
- Chen G, Højer AM, Areberg J, Nomikos G (2018) Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin Pharmacokinet 57(6): 673-686.
- Sanchez C, Asin KE, Artigas F (2015) Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol Ther 145: 43-57.
- Spina E, Santoro V (2015) Drug interactions with vortioxetine, a new multimodal antidepressant. Riv Psichiatr 50(5): 210-215.
- Vortioxetine: Interactions. (2018) Accessed on: September 26, 2018. Available online at: https://www.drugbank.ca/drugs/DB09068; U.S. Government. Drug-Drug Interactions. Accessed on: September 26, 2018. Available online at: http://m.usgovxml.com/DrugInt.aspx?dn=SINEMET
- Ladefoged LK, Munro L, Pedersen AJ, Balle T, Andersen BB, et al. (2018) Modeling and mutational analysis of the binding mode for the multimodal antidepressant drug vortioxetine to the human 5-HT3A receptor. Mol Pharmacol 94(6): 1421-1434.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Allergy Research (ISSN:2642-326X)

