861
Views & Citations10
Likes & Shares
Menopause may be considered as a factor that increases the risk of developing rheumatoid arthritis in women. The study aimed to determine possible changes in serum levels of anti-citrullinated protein antibody (ACPA), rheumatoid factor (RF) and alkaline phosphatase (ALP) in menopausal women as possible risk factors for developing rheumatoid arthritis. The subjects comprised of hundred females; fifty pre-menopausal women and fifty menopausal women. Blood specimen was collected from the subjects and serum extracted for the estimation of ACPA which was determined by Enzyme-linked Immunosorbent Assay (ELISA) technique; alkaline phosphatase was determined spectrophotometrically and rheumatoid factor was determined using the latex- slide method. The results showed that the number of women who were positive for both ACPA and RF were significantly higher (p<0.01) in menopausal than pre-menopausal women. There was no significant increase (p>0.05) in ACPA and ALP levels in menopausal women compared with pre-menopausal women. However, significant positive relationships (p<0.01) were obtained when ACPA and ALP levels were correlated in both pre- menopausal and menopausal women. The study concluded that increased levels of ACPA, ALP and RF may be suggestive of the onset of rheumatoid arthritis in menopause. Therefore, ACPA, serum ALP and RF could be used as markers for the diagnosis of rheumatoid arthritis and monitoring of disease progression.
Keywords: Anti-citrullinated protein antibody, Rheumatoid factor, Alkaline phosphatase, Menopausal women
INTRODUCTION
Menopause may be associated with significant changes such as chromosomal abnormality or autoimmune disorder [1]. Early menopause may be a factor that increases the risk of developing rheumatoid arthritis and menopausal period is associated with the most significant levels of joint damage and disability amongst women with rheumatoid arthritis [2]. Rheumatoid arthritis is a systemic autoimmune disorder and inflammatory disease [3]. At menopause, estrogen level decreases and may cause symptoms of rheumatoid arthritis (RA) to aggravate. Cardiovascular diseases are also associated with RA and the inflammatory pathways involved include cytokines, immune complexes, acute phase reactants [4]. Rheumatoid factor could be involved in the pathogenesis of rheumatoid arthritis and could enhance immune complex formation leading to bone damage [5-7]. Rheumatoid factor may also be positive in patients who have systemic lupus erythematosus, polymyositis and syphilis [8].
Anti-Citrullinated Protein Antibody (ACPA) is primarily associated with a progressive course of rheumatoid arthritis [9]. Citrullination normally occurs in cells undergoing apoptosis; hence, citrullinated proteins are cleared from the body and therefore not encountered by the body’s immune system [10]. ACPA and bone destruction are associated with each other implying that ACPAs may directly mediate bone destruction in the absence of RA- associated factors [11]. Anti-Citrullinated protein antibodies are detectable in patients at risk of Rheumatoid Arthritis long before the onset of the disease and ACPA has been established as the most specific serological marker for rheumatoid arthritis [12]. ACPA concentration has been shown to increase as the disease progresses [10] and its production depends on the genetic background of the patient. Some researchers have associated early onset of menopause with increased risk of seronegative- rheumatoid arthritis, therefore other factors relating to menopause may be relevant for the development of rheumatoid arthritis in the presence of anti-citrullinated protein antibody [13].
Alkaline phosphatase (ALP) comprises of a group of enzymes that catalyze the hydrolysis of phosphate esters in an alkaline environment, generating an organic radical and inorganic phosphate [14]. In rheumatoid arthritis, there is increased bone turnover as well as bone formation and bone resorption may affect bone density. It has been shown that the estimation of serum alkaline phosphatase may be a biomarker for the diagnosis and prognosis of rheumatoid arthritis [15]. Apart from being used as a biomarker for RA, it may also be useful in the stratification and observing RA patients in routine or clinical trials [16]. Therefore, since anti-citrullinated protein antibody are autoantibodies and a major risk factor for bone destruction at early phase of rheumatoid arthritis, the study set to determine possible changes in anti-citrullinated protein antibody, rheumatoid factor and alkaline phosphatase in menopausal women as risk factors for developing rheumatoid arthritis and determine if there is relationship between the parameters for the diagnosis of rheumatoid arthritis in pre- and menopausal women.
MATERIALS AND METHODS
Study area
The study was conducted in Ido-Ekiti, Ekiti State while laboratory analysis was carried out in the Department of Medical Laboratory Science, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria.
Study subjects
The subjects comprised of hundred women; fifty pre-menopausal women and fifty menopausal women recruited from Ado and Ido Ekiti using convenient random sampling. The women whose ages fall below 50 years and were menstruating was grouped as pre-menopausal women while those above 50 years who have stopped menstruating were categorized as menopausal women. Women below the age of 20 years, pregnant women, lactating mothers, women above the age of 65 years, women on any type of medication or any disease condition were excluded from the study. Five milliliters of venous blood were collected from the cubital fossa of the forearm using needle and syringe and dispensed into a plain bottle. The samples were separated by centrifugation at 3000 rpm for 5 min to extract the serum. The serum samples were then stored at -20°C until analysis.
Ethical approval
Ethical approval was sought for and obtained from the Federal Teaching Hospital, Ido-Ekiti, Ekiti State and the College of Medicine and Health Sciences, Afe Babalola University Ado-Ekiti, Ekiti State. The nature and purpose of the research was explained to each participant and the participants were assured of confidentiality. Those who participated in the study did so voluntarily.
Principle of the assay
Determination of serum alkaline phosphatase using spectrophotometric method
Phenol released by enzymatic hydrolysis from phenyl-phosphate under defined conditions of time, temperature and pH is estimated spectrophotometrically.
Determination of serum rheumatoid factor
Rheumatoid factor was determined using the latex- slide method as described by the manufacturer (Biosystems from S.A. Barcelona, Spain); [19].
Principle of the assay
Serum rheumatoid factor (RF) causes a visible agglutination on the slide of a suspension of latex particles coated with human gamma-globulin.
Statistical analysis
Results obtained were subjected to statistical analysis using the software package for social sciences (SPSS) version 21.0 (SPSS Inc. Chicago, Illinois, USA). All parameters were expressed as mean ± standard deviation (SD). The student’s t-test, Chi2, and correlations of the parameters were carried out. Statistical significance was set at p <0.05.
RESULTS
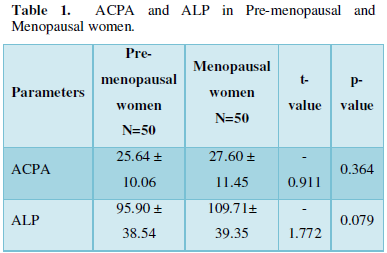
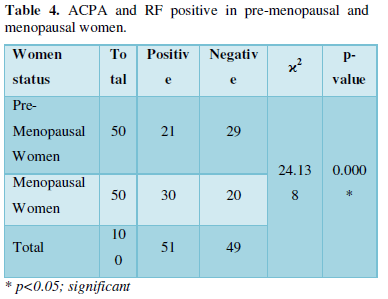
Table 4 shows the results of ACPA and RF positive in pre-menopausal and menopausal women. Significant increase was observed when those who were positive for both ACPA and RF in menopausal women were compared pre-menopausal women.
DISCUSSION
Menopause exhibits important roles in the physical and emotional aspects in women [20]. Menopausal period is associated with significant levels of joint damage as well as disability amongst women with rheumatoid arthritis [2]. This research assessed the possible changes in serum levels of anti-citrullinated protein antibody (ACPA), rheumatoid factors (RF) and alkaline phosphatase (ALP) in pre-menopausal and menopausal women.
It was observed that serum levels of ACPA were higher (although not significant) in menopausal women compared with pre- menopausal women. This supports the findings of other researchers [21,22] whose reports showed significant increase when both groups were compared. This has also been supported by the works of a previous study [13] which showed that ACPA levels increase with age, and menopausal women have a higher ACPA-positive result than pre-menopausal women. The causal factor could be associated with hormonal changes during menopause which are linked with increase of pro-inflammatory cytokines resulting in development of ACPAs. This has been proved to occur in menopause as a result of failure in ovarian activity, low or reduced estrogen levels as well as increased bone turnover.
The higher levels of ALP observed in menopausal women also support the findings of Sajjanar and Sajjanar [23] where significant increase in ALP was reported in menopausal women compared with pre-menopausal women. This finding is possible because bone mass decreases with age and the rate of bone remodeling also increases with menopause. Higher levels of ALP in menopausal women have also been reported by other researchers [24- 26]. This may be as a result of estrogen deficiency in menopause that induces the synthesis of cytokines by osteoblasts, monocytes and T-cells and therefore osteoblastic activity is increased when bone resorption is stimulated. Furthermore, differentiation into bone-reabsorbing osteoclasts can be promoted when osteoclast precursor cells are bound to anti-citrullinated protein antibodies.
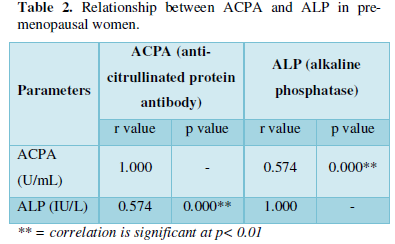
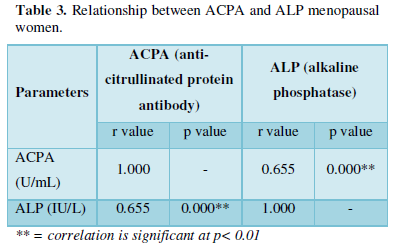
Significant positive correlations were observed between ALP and ACPA in both pre-menopausal and menopausal women. This is in line with a study carried out by Kocijan et al [27] where it was reported that bone loss in ACPA-positive individuals may occur earlier in women before the onset of clinical disease.
There was significant increase observed when women who were positive for both ACPA and RF in menopausal stage were compared with women in pre-menopausal stage. This agrees with the works [28, 29] who found that positive ACPA and RF result was higher in menopausal women who had menopause at early age. A previous study [30] also supported the findings of increased RF-positive in menopausal women compared with pre-menopausal women. This has been attributed to changes in hormonal factors such as estrogen and progesterone.
CONCLUSION
This study showed possible changes that could occur in the serum levels of anti-citrullinated protein antibody (ACPA), alkaline phosphatase (ALP) and rheumatoid factor (RF) in pre- and menopausal women. Menopausal women had higher (although not significant) serum levels of ACPA, ALP and RF compared with pre-menopausal women. The study concluded that increased levels of ACPA, ALP and RF may be suggestive of the onset of rheumatoid arthritis in menopause. Therefore, ACPA, serum ALP and RF could be used as markers for the diagnosis of rheumatoid arthritis and monitoring of disease progression.
ACKNOWLEDGEMENT
The authors acknowledge the staff members of Department of Chemical pathology of Federal Teaching Hospital, Ido-Ekiti, Ekiti State, Nigeria for their assistance during sample collection.
COMPETING INTERESTS
The authors declare that there is no competing interest.
- Dalal PK, Agarwal M (2015) Postmenopausal syndrome. Indian J Psychiatry 57(2): 222-232.
- Tracey G (2017) Diagnosis and management of rheumatoid arthritis. Prescribing in Practice 24: 13-17.
- Attur M, Mignatti P, Han T, Attur MG (2019) Extracellular Vesicles Biology and its Emerging Role in Osteoarthritis and Related Arthritides. Rheumatology (Sunnyvale) 9(2): 1000254.
- Peters MJL, Symmons DPM, McCarey D, Dijkmans BAC, Nicola P, et al. (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69: 325-331.
- Mannik M (1992) Rheumatoid factors in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl 32: 46-49.
- Song WK, Kang EH (2010) The pathogenic role of rheumatoid factor in rheumatoid arthritis. Int J Clin Rheumatol 5(6): 651-658.
- Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, et al. (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies.. Bone Res 6:
- Paul BJ, Kandy HI, Krishnan V (2017) Pre-rheumatoid arthritis and its prevention. Europ J Rheumatol 4: 161-165.
- Szekanecz Z, Soos L, Szabo Z, Fekete A, Kapitany A, et al. (2008) Anti-citrullinated protein antibodies in rheumatoid arthritis: As good as it gets? Clin Rev Allergy Immunol34(1): 26-31.
- Moeez S, John P, Bhatti A (2013) Anti-citrullinated protein antibodies: Role in pathogenesis of rheumatoid arthritis and potential as a diagnostic tool. Rheumatol Int 33(7): 1669-1673.
- Behrens F, Koehm M, Greger G, Thaci D, Burkhardt H (2016) Anti-citrullinated protein Antibodies are linked to erosive disease in an observational study of patients with Psoriatic arthritis. Rheumatology 55(10): 1791-1795.
- Wegner N, Wait R, Sroka A, Eick S, Nguyen K-A, et al. (2010) Peptidylarginine deaminase from porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 62: 2662-2672.
- Alpizar-Rodriguez D, Muller R, Dudler J, Ciurea A, Kyburz D, et al. (2016) Menopause is a predictor for the development of anti-citrullinated protein antibodies in women at risk for rheumatoid arthritis. Ann Rheum Dis 75(2): 376-378.
- Chandrakar BL, Sharma HC, Chandrakar KS (2017) Activity of serum alkaline phosphatase in rheumatoid arthritis for diagnosis and management. Int J Med Res Pro 3(3): 281-284.
- Dubey A, Hijam D, Debnath S, Haokip L, Devi TI (2017) Study of serum alkaline phosphatase level in rheumatoid arthritis. Int J Med Res Pro 3(3): 318-321.
- Najeeb Q, Aziz R (2015) Comparison of alkaline phosphatase, lactate dehydrogenase and acid phosphatase levels in serum and synovial fluid between patients with rheumatoid arthritis and osteoarthritis. Int J Sci Res 4(4): 1069-1075.
- Samanci N, Ozdem S, Akbas H, Mutlu D, Gultekin M, et al. (2005) Diagnostic value and clinical significance of anti-CCP in patients with advanced rheumatoid arthritis. J Natl Med Assoc 97(8): 261-286.
- Moss DW, Baron DN, Walker PG, Wilkinson JH (1971) Standardization of clinical enzyme assays. J Clin Pathol 24: 740-743.
- Sager D, Wernick RM, Davey MP (1992) Assays for rheumatoid factor: A review of their utility and limitations in clinical practice. Lab Med 23: 15-18.
- Kangau H (2017) Menopause: Health promotion strategies from a nursing perspective. Arcada/ Basic Sexology 14: 23-26.
- Orellana C, Saevarsdottir S, Klareskog L, Karlson E, Alfredsson, L, et al. (2015) Postmenopausal hormone therapy and the risk of rheumatoid arthritis: Results from the Swedish EIRA population-based case-control study. Eur J Epidemiol 30(5): 449-457.
- Mosaad Y, Hammad E, Fawzy I, Marouf S, AL-Harrass M, et al. (2015) Value of anti-cyclic citrullinated peptide and rheumatoid factor antibodies in relation to rheumatoid arthritis: Association with osteoporosis. Int J Adv Res 3(8): 18-32.
- Sajjanar D, Sajjanar S (2014) Study of serum alkaline phosphatase, calcium and urinary hydroxyproline as bone biomarkers in postmenopausal women. Int J App Basic Med Res 4(1): 223-229.
- Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P (2014) Correlation of common biochemical markers for bone turnover, serum calcium and alkaline phosphatase in postmenopausal women. Malays J Med Sci 21(1): 58-61.
- Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, et al. (2015) Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res 27(4): 413-418.
- Pardhe B, Pathak S, Bhetwal A, Ghimire S, Shakya S, et al. (2017) Effect of age and estrogen on biochemical markers of bone turnover in postmenopausal women: a population-based study from Nepal. Int J Womens Health 9: 781-788.
- Kocijan R, Harre U, Schett G (2013) ACPA and bone loss in rheumatoid arthritis. Curr Rheumatol Rep 15(10): 366-370.
- Wong L, Huang W, Xiong J, Boire G, Haraoul B, et al. (2015) Effect of age at menopause in women with early rheumatoid arthritis. Am College Rheumatol 67(5): 616-623.
- Biscaldi L (2017) Menopause and rheumatoid arthritis risk. Rheumatol Advisor 5(2): 44-54.
- Pikwer M, Nilsson J-A, Bergstrom U, Jacobsson LTH, Turesson C (2012) Early menopause and severity of rheumatoid arthritis in women older than 45 years. Arthritis Res Ther 14(4): 190-192.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Rheumatology Research (ISSN:2641-6999)

