202
Views & Citations10
Likes & Shares
Oral thin film was first developed in 1970’s as a novel dosage form and film were launched in 2004 for systemic drug delivery [2]. Fast dissolving oral disintegrating film in which drug is release instantly from the dosage form i.e. within 1 min and rapidly absorb via mouth and it reaches quickly into blood circulation hence maximum bioavailability is been achieved [3].
A dosage form which dissolves or disintegrates quickly in the oral cavity, resulting in solution or suspension without the need for the administration of water, is known as an oral fast dissolving dosage form. Mouth Dissolving Film (MDF) is also known as Fast dissolving film, Quick dissolving film, Rapid dissolving film, Oral thin film (OTF), Orally Dissolving Films (ODF).
ODFs are commonly prepared using hydrophilic polymers enabling rapid dissolution upon contact with saliva. A typical ODF is usually equal to the size of a postage stamp. Such type of dosage form having good bioavailability as comparatively than the conventional dosage form [4] (Figure 1).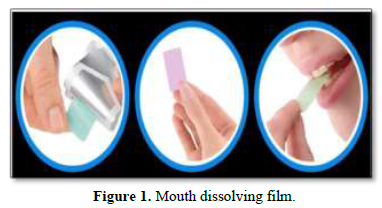
Special features of Mouth Dissolving Film [5]
- Available in different size and shape
- Optimal muco adhesion
- Skinny elegant films
- Unobstructive
- Fast dissolving
- Rapid disintegrating
- Quick release
Merits of Mouth Dissolving Film [6]
- Greater surface area promotes fast disintegration and dissolution in the oral cavity.
- Oral films are flexible and less fragile. Hence, there is ease of transportation and during consumer handling and storage.
- Accurate and precise dosing.
- No risk of choking
- Good mouth feels
- Improved patient compliance
- No need of water for swallowing gives better acceptability amongst the dysphagic patients
- Convenient dosage form so, can be consumed at any place and anytime of the individual.
- Enhanced oral bioavailability of molecules by avoiding first pass effect.
- Bypassing the first pass effect leads to reduction of dose which can lead to reduction in side effects associated with the molecules.
- Mouth Dissolving Films are typically the size of a postage stamp and disintegrate on a patient’s tongue in a matter of seconds for the rapid release of one or more APIs.
Demerits
- Drugs which are unstable at buccal pH cannot be administrated.
- Taste masking is needed.
- Dose uniformity is a big challenge in formulation.
- Low dose of drug required.
- Costly packaging of oral film
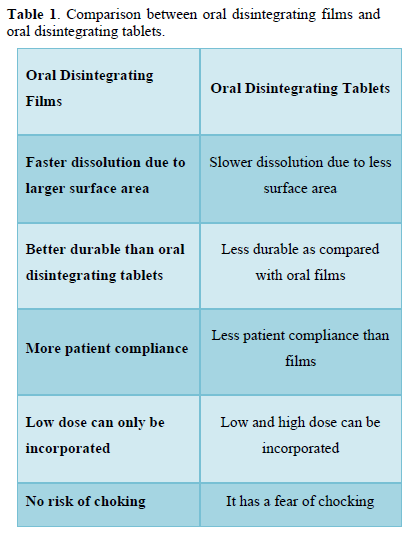
Comparison Between Oral Disintegrating Films and Oral Disintegrating Tablets [7] (Table 1)
Ideal Characteristic of a suitable Drug Candidate [8]
- The drug should have pleasant taste.
- The drug should have low dose up to 30 mg.
- The drugs with low and moderate molecular weight are preferable.
- The drug should have good stability and solubility in water as well as in saliva.
- Drug should be partially unionized at the pH of oral cavity.
- Drug should have the ability to permeate oral mucosal tissue.
Composition of the System [9,10]
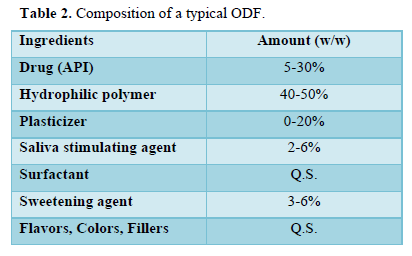
ODFs are fast disintegrating thin films having an area ranging from 5 to 20 cm2 in which drug is incorporated with hydrophilic polymer. API can be incorporated up to 30 mg along with other additives like plasticizers, colorants, sweeteners, taste masking agents etc. (Table 2).
Primary concerns when manufacture Mouth dissolving films [6,11-14]
Selection of the API: The selection of active pharmaceutical ingredient depends on the potency of drug, dose and its therapeutic efficacy. Anti-allergic, antihistaminic, anti-Parkinson, sleeping aids and analgesic class of drugs are most suitable for preparation of MDF.
Selection of the Film formers: The film should be strong enough to be physically handled and the robustness of the film depends on the type of polymer used. The film must also dissolve easily in saliva or water to be effective immediately. In order to obtain a good formulation, at least 40-50% w/w polymer should be present in the formulation. Pullulan, gelatin, HPMC and HPC are the most commonly used polymers in the film formulation.
Plasticizer: The plasticizer provides effective plasticity to the MDF formulation. Care must be taken for determining the concentration of the plasticizer. The concentration of the plasticizer usually ranges from 0-20% w/w. PEG, glycerol, diethyl phthalate, triethyl citrate, tributyl citrate, etc. are the widely used plasticizer in MDF [12].
Taste masking: Taste masking is a prerequisite in the case of an oral formulation. Both natural and artificial sweeteners are used to enhance taste and are designed to dissolve and disintegrate in the oral cavity. The classic sources of sugar are sucrose, fructose, glucose and dextrose. Saccharin, sucralose and aspartame fall into the category of artificial sweeteners.
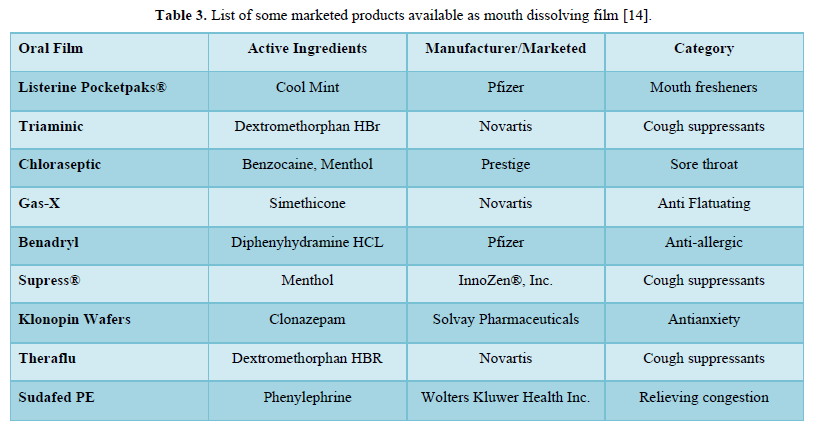
Saliva stimulating agent: Salivary stimulants are generally acidic in nature, stimulating the production of saliva in the buccal cavity and subsequently promoting the disintegration of MDF. Some commonly used saliva stimulants are citric acid, malic acid, tartaric acid, ascorbic acid, and lactic acid [13] (Table 3).
Approaches for manufacturing of mouth dissolving films (Figure 1)
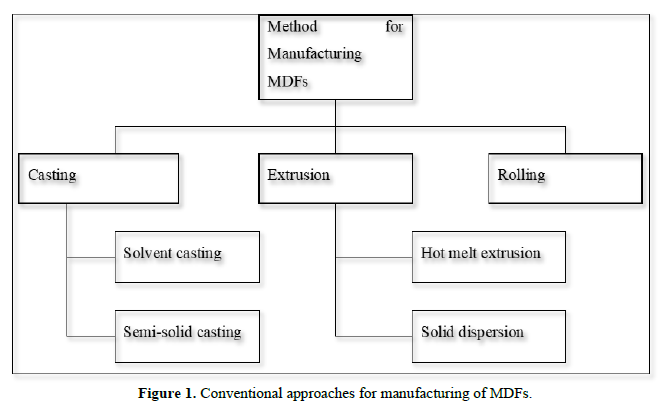
METHOD OF PREPARATION OF FAST DISSOLVING FILM [14-22]
Following methods can be used for manufacturing the mouth dissolving film:
- Solvent casting
- Hot-melt extrusion
- Semisolid casting
- Solid dispersion extrusion
- Rolling
- Spray technique
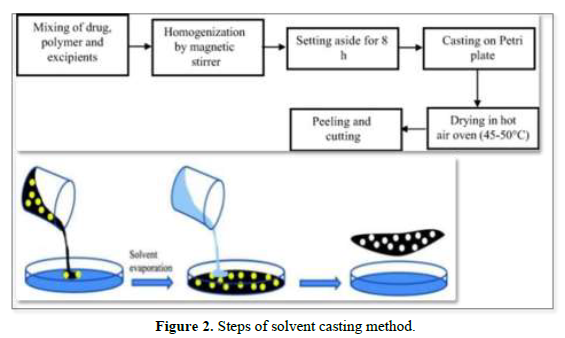
Solvent Casting Method
Solvent casting is the most commonly used method for the preparation of MDFs using water-soluble excipients, polymers, and drugs that are dissolved in deionized water; as a result, a homogeneous mixture is obtained by applying high shear forces generated by the shear processor. Then the prepared solution is poured into a petri plate and the solvent is dried by exposure to high temperature to obtain good quality films [19,20] (Figure 2).
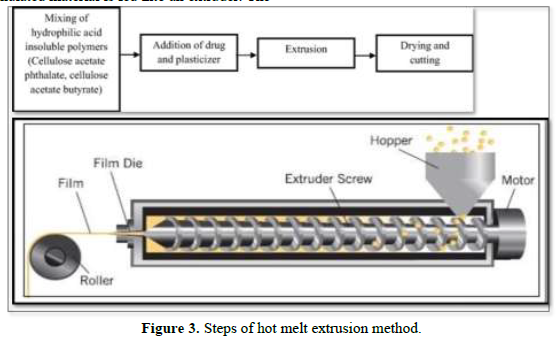
Hot-melt extrusion
In this method, the drug is mixed with a carrier in solid form and the dried granulated material is fed into an extruder. The screw speed should be 15 rpm. In the extruder, the solid carrier and drug are melted and placed in dies and then cut to the desired size and shapes [21] (Figure 3).
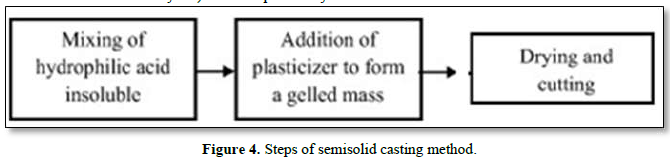
Semisolid casting
In this method, a solution of water-soluble and film-forming polymer is prepared. The resulting solution is added to a solution of an acid-insoluble polymer (e.g. cellulose acetate phthalate and cellulose acetate butyrate) which is previously prepared in ammonium or sodium hydroxide. An appropriate amount of plasticizer is added to obtain a gel mass. The prepared gel mass is cast into films or strips using a controlled heat source. Film thickness is controlled between 0.015-0.05 inches [20] (Figure 4).
Solid dispersion extrusion
The term solid dispersion refers to the dispersion of one or more active ingredients in an inert carrier in a solid state in the presence of amorphous hydrophilic polymers. In this method, the drug is dissolved in a suitable solvent, then the solution is incorporated into a melt of polyols, such as polyethylene glycol, and optionally, the unit surface area of the solid dispersion is formed into films using matrices [22] (Figure 5).

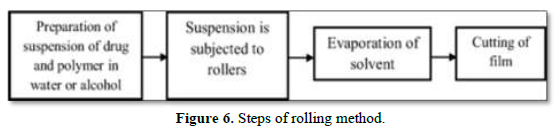
Rolling
In this method, a suspension of drug and polymer containing water or alcohol as a solvent is prepared and exposed to a roller. The prepared suspension is added on drum for solvent evaporation and cut to the desired size and shape (Figure 6).
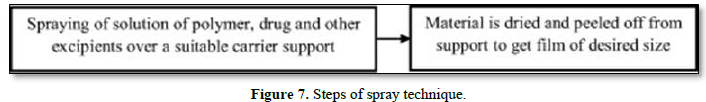
Spray Technique
Drug, polymers and all other excipients are dissolved in a suitable solvent to form a clear solution. This clear solution is then sprayed onto a suitable material such as glass, polyethylene film of non-siliconized Kraft paper, or Teflon foil. Allow to dry and peel off the backing to obtain the desired size film [21] (Figure 7).
EVALUATION PARAMETERS [23-29]
Thickness
It can be measured with a micrometer screw gauge or Vernier caliper. It can be checked at five different points with a calibrated digital micrometer for content uniformity and uniform film thickness.
Dryness test/tack test
This test is performed to determine the ability of the film to adhere to a piece of paper pressed between the strips. The tenacity with which a film adheres to a piece of paper or other accessory pressed between the films is known as tack. There are almost eight stages in the film drying process, which are identified as dry to the touch, dry to drag, dry hard, dry to the touch, dustless, dry-through, non-sticky and non-printing dry. These tests are primarily used to evaluate the dryness of films in the coatings industry, but are also applicable to the evaluation of rapidly disintegrating films in the mouth. Dryness or stickiness test can also be done with some newly invented tools.
Tensile strength
Tensile strength is the maximum stress applied to the point at which the strip specimen breaks. It is calculated as the applied breaking load divided by the cross-sectional area of the strip as shown in the equation below:
Percent elongation
When tension is applied, the strip pattern stretches and this is often called deformation. The deformation is basically the deformation of the strip divided by the original dimension of the sample. The percentage of elongation is quantitatively related to the amount of plasticizer used in the film formulation. Increased plasticizer concentration in the film generally results in increased strip elongation.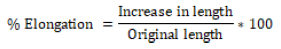
Tear resistance
The tear resistance of a film is a complex function of its ultimate resistance to rupture. The maximum force required to tear the film is measured as the tear resistance value. It is expressed in Newton or Pound-Force.
Folding endurance
The folding endurance is another procedure for estimating the mechanical properties of the film. The folding endurance is set by repeatedly folding the strip in the same place until the strip breaks. The folding resistance value is the number of times the film is folded without breaking. A higher folding endurance value indicates greater mechanical strength of the film. There is a direct relationship between the mechanical strength and the folding endurance of the films. Since the mechanical strength is controlled by the concentration of the plasticizer, it is clearly evident that the concentration of the plasticizer also indirectly affects the folding endurance value.
Surface pH of film
The surface pH of the films is determined by placing a film on the surface of 1.5% w/v agar gel and then placing pH paper (pH range 1-11) on the films. The color change of the pH paper is observed and reported.
Swelling property
Film swelling studies are performed using a simulated saliva solution. The initial weight of the film is determined and placed in a pre-weighed stainless-steel wire mesh. This film containing the screen is then immersed in a simulated saliva solution. The increase in weight of the film is recorded at constant predetermined time intervals until no more weight increases.
The degree of swelling is calculated using parameters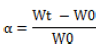
Where, Wt is weight of film at time t and W0 is weight of film at time zero
Transparency
The transparency of the strip is determined using a UV-spectrophotometer. This test is performed for the visual appearance of the formulation. Film preparations are cut into rectangular shapes and placed on the inside of the photometric cell. The transmittance of the film is determined at a wavelength of 600 nm. Film transparency is calculated as follows: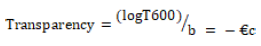
Where T600 is the transmittance at 600 nm and b is the film thickness (mm) and c is concentration.
Assay/ Content uniformity
This is determined by the standard test methodology represented for the specific API in the standard pharmacopoeia. Content uniformity is determined by the estimation of API content in individual strips. The limit for content uniformity is 85-115%.
Disintegration time
The disintegration apparatus specified in the official pharmacopoeias is used to determine the film disintegration time. Normally, disintegration time is a function of film composition, as it varies with composition and generally ranges from 5 to 30s. No official guidelines are available for determining the disintegration time of orally rapid disintegrating films. There are two ways to determine the film disintegration time:
- Slide frame method: A drop of distilled water is poured onto the film clamped into slide frames placed on petri dish. Time taken by the film to dissolve is noted.
- Petri dish method: A film is placed onto 2 ml distilled water taken in petri dish. Time taken by the film to dissolve completely is considered as the disintegrating time.
Dissolution test
Dissolution testing can be performed using a standard basket or paddle apparatus described in the pharmacopoeia. Basically, the dissolution medium will be selected according to the sink conditions and the highest API dose. Many times, the dissolution test can be difficult due to the tendency of the strip to float on the dissolution medium when a paddle apparatus is used. It is carried out in 0.1N HCl and phosphate buffer pH 6.8 dissolution media. The temperature is maintained at 37 ± 0.5°C and the rotation speed is usually set at 50 rpm. Samples of the dissolved drug are taken at predetermined time intervals and analyzed using a UV-spectrophotometer
Visual inspection and surface morphology
Visual inspection of the prepared mouth dissolving film provides information on color, homogeneity and transparency. Scanning electron microscopy is performed for surface morphology. The absence of pores and the uniformity of the surface testify to the good quality of the films.
STORAGE AND PACKAGING [29]
Fast dissolving films can be packaged using individual pouches, multi-unit blister cards, multi-unit dispensers, and endless roll dispensers. There are certain proprietary packaging systems for fast-dissolving films, such as Rapid card by Labtech and Core-peel by Amcor flexible. The rapid card is the same size as a credit card and can hold three films on each side. Each dose can be taken individually.
CONCLUSION
This review shows that mouth dissolving films are one of the new approaches in the field of pharmaceutical sciences. MDFs have improved patient acceptability and compliance without the risk of choking associated with better safety and efficacy compared to conventional dosage forms. The main idea behind the MDF formulation is to cope with the difficulty in swallowing conventional oral dosage forms in pediatric, geriatric and psychiatric patients with dysphasia. Currently, MDFs are widely available for hypertension, acidity, allergy, pain, etc., reflecting their importance. The main advantages of such a dosage form are their administration without the use of water, meeting the need of the target population seeking convenience in drug administration, along with bypassing hepatic metabolism, which in turn leads to an improved therapeutic response.
- Vishwakarma DK, Banerjeer A (2018) Mouth Dissolving Film: An approach to novel drug delivery system-A Review. Acta Biomedica Scientia 5(1): 29-36.
- Reza KH, Chakraborty P (2016) Recent industrial development in Oral Thin Film Technology: An Overview. PharmaTutor 4(8): 17-22.
- Shubham PR, Raosaheb SS, Aniket PJ, Suresh PD, Vaishnavi PB, et al. (2021) A Review On: Mouth Dissolving Film. Int J of Pharm Res and App 6(3): 370-378.
- Okabe H, Suzuki E, Sugiur Y, Yanagimoto K, Takanashi Y, et al. (2008) Development of easily Swallowed film formulation. Int J Pharm 355: 62-66.
- Patel PG (2010) To develop and evaluate oral dissolving film of acetaminophen. Master of Sci. Thesis, Long Island University.
- Gavaskar B, Vijayakumar S, Sharan G (2010) Overview on fast dissolving films. Int J Pharm Pharm Sci 2(3): 29-33.
- Perioli L, Ambrogi V, Angelici F, Ricci M, Giovagnoli S, et al. (2004) Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release 99 (1): 73-82.
- Bhyan B, Jangra S, Kaur M, Singh H (2011) Orally fast dissolving films: Innovations in formulation and technology. Int J of Pharma Sci Rev Res 9(2): 50-57.
- Kulkarni N, Kumar LD (2003) U.S Patent, Fast dissolving orally consumable films containing an antitussive and a mucosa coating agent.2003/206942.
- Arya A, Chandra A, Sharma V, Pathak K (2010) Fast dissolving oral films: An innovative drug delivery system and dosage form. Int J Chem Tech Res 2: 576-583.
- Mashru RC, Sutariya VB, Sankalia MG, Parikh P (2005) Development and evaluation of fast dissolving film of salbutamol sulphate. Drug Dev Ind Pharm 31: 25-34.
- Bala R, Pawar P, Khanna S, Arora S (2013) Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig 3: 67-73.
- Siddiqui MN, Garg G, Sharma PK (2011) A short review on a novel approach in oral fast dissolving drug delivery system and their patents. Adv Biol Res 5: 291-303.
- Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, et al. (2016) Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm J 24(5): 537-546.
- Malke M, Shidhaye S, Kadam VJ (2007) Formulation and evaluation of Oxacarbazine fast dissolve tablets. Indian J Pharm Sci 69(2): 211-214.
- Chandra A, Sharma V, Pathak K (2010) Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form. Int J Chem Tech Res 2(1): 576-583.
- Lydzinski S, Manegold T, Solarek D, Tsai J, Puglisi C (2003) Film containing starch, U.S Patent 2003/0099692.
- Wening K, Breitkreutz J (2011) Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int J Pharm 404(1-2): 1-9.
- Choudhary DR, Patel VA, Chhalotiya UK, Patel HV, Kundawala AJ (2012) Development and characterization of pharmacokinetic parameters of fast-dissolving films containing levocetirizine. Sci Pharm 80: 779-787.
- Thakur RR, Rathore DS, Narwal S (2012) Orally disintegrating preparations: recent advancement in formulation and technology. J Drug Deliv Therap 2(3): 87-96.
- Panda BP, Dey NS, Rao MEB (2012) Development of innovative orally fast disintegrating film dosage forms: A review. Int J Pharm Sci Nanotechnol 5: 1666-1674.
- Joshi P, Patel H, Patel V, Panchal R (2012) Formulation development and evaluation of mouth dissolving film of domperidone. J Pharm Bioallied Sci 108: 9-17.
- Dixit RP, Puthli SP (2009) Oral strip technology: Overview and future potential. J Cont Rel 139: 94-97.
- Browhn GL (1956) Formation of film from polymer dispersions. J Polym Sci 22: 423-234.
- Patil PC, Shrivastava SK, Vaidehi S, Ashwini P (2014) Oral fast dissolving drug delivery system: A modern approach for patient compliance. Int J Drug Reg Affairs 2(2): 49-60.
- Patel AR, Prajapati DS, Raval JA (2010) Fast dissolving films (FDFs) as a newer venture in fast dissolving dosage forms. Int J Drug Dev Res 2: 232-246.
- Bala R, Pawar P, Khanna S, Arora S (2013) Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig 3(2): 67-76.
- Thakur RR, Narwal S, Rathore DS (2012) Orally disintegrating preparations: Recent advancement in formulation and technology. J Drug Deliv Ther 2(3): 87-96.
- Jung H, John F (1997) Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J Plastic Film Sheeting 13: 87-297.









