1292
Views & Citations292
Likes & Shares
Background: The aim of this study was to evaluate the therapeutic effect of pilescure in hemorrhoids induced Sprague Dawley male rats.
Methods: Total 30 male Sprague Dawley rats were selected and randomized into three groups of ten animals each. Group I was control, whereas group II and group III were hemorrhoid and hemorrhoid plus pilescure treated groups. Hemorrhoid was induced in group II and group III by application of 6.0% croton oil preparation. At the end of experiment, rectoanal weight was recorded and blood sample was for measurement of lipid peroxidation, white blood cells, nitric oxide and C-reactive protein levels along with some antioxidants enzyme activities and cytokine parameters in all groups. The histological analysis was performed in recto-anal tissue of all groups.
Results: The findings revealed that the LD50 of drug was found more than 2000 mg/kg in acute toxicity study. No clinical sign of toxicity, mortality were found up to 2000 mg/kg body weight. In efficacy study, there was a statistically significantly (p<0.0001) reduction in recto-anal weight, recto-anal coefficient and the levels of WBC, neutrophil, CRP, NO, lipid peroxidation and cytokines parameters along with increased stool frequency, level of hemoglobin, platelet count and some antioxidant enzyme activities in pilescure treated group as compared with hemorrhoids induced group and these levels were comes back to control group after treatment with drug for three weeks. The histopathological finding also clearly showed that inflammatory cells were found low after treatment with drug as compared to hemorrhoid induced group.
Conclusion: The study concluded that pilescure is most effective ayurvedic medicine for reduction of inflammation along with increase antioxidant enzymes and hematological parameters and decrease oxidative stress during hemorrhoids condition.
Keywords: Pilescure, Hemorrhoids, Hematological and oxidative stress, Antioxidant and anti-inflammatory, Recto-anal tissue
Abbreviations: RAC: Recto-Anal Coefficient; CRP: C-Reactive Protein; WBC: White Blood Cells MDA: Malonaldialdehyde; TNF-α: Tumor Necrosis Factor-α; IL-β: Interleukin-β; IL-6: Interleukin-6; NO: Nitric Oxide; SOD: Superoxide Dismutase; GR: Glutathione Reductase; GPx: Glutathione Peroxidase; GSH: Reduced Glutathione
The treatment of hemorrhoids in modern medicine is still in infancy. There are many topical agents or suppositories are available for treatment of hemorrhoids, there is little evidence to support and their use [12,13]. Now a day's extensive research is going on in the field of ayurveda for utilizing the natural resources for treating hemorrhoids. Various medicinal plants are used in the treatment of hemorrhoids. Pilescure is one of the ayurvedic medicines which consist of poly-ingredients. The ingredients of pilescure are Abhaya (Terminalia chebula), Nagkesar (Mesua ferrea), Arand mool (Ricinus communis) and Purified alum (Sudhasphatika). These individual ingredients have been reported as an effective for management of rectal pain, rectal bleeding, anti-inflammatory and constipation, etc. [14-17]. Terminalia chebula is one of them which is also cited in Charaka's Arshoghna Mahakashaya [18]. This drug is manufacture under the GMP (Good Manufacturing Practices) condition. Its botanical identification, quality parameters and ayurvedic criteria are complied with pharmacopoeia standard. So the purpose of this study was to investigate the protective effect of polyherbal drug pilescure in experimental model of croton oil induced hemorrhoid in Sprague Dawley rats.
MATERIALS AND METHODS
Chemicals
All biochemical such as reduced glutathione, NADPH, thiobarbutric acid, SDS, Croton oil, used in the present study were procured from Sigma, St. Louis, MO, USA. Other biochemical and chemicals (butanol, pyridine and diethyl ether, etc.) purchased locally were of HiMedia and analytical grade.
Drug
Pilescure drug was obtained from sponsor Lavanaya Pharmacy Pvt. Ltd., Lucknow, to conduct study on animals. Total 20 capsules were obtained from sponsor. The purity and chemical analysis is not part of this study and it was responsibility of sponsor. The drug concentration was 500 mg/capsules.
Acute toxicity
An acute oral toxicity study was performed by method of Parasuraman [19]. In acute oral toxicity, we followed the acute toxic category method. Total three Sprague Dawley male rat (n=3) were selected at different dose levels. The animals were kept overnight with access to water but not food, after which the pilescure drug was administered orally at a different dose level of 500, 1000 and 2000 mg/kg body weight and the animals were monitored for clinical sign of toxicity, mortality for 24 h. If any mortality was observed in 2 out of 3 animals, then the dose administered was identified as a toxic dose. If mortality was observed in one animal, then the same dose was repeated again to confirm the toxic dose.
Experimental design
The study was carried out in animals after getting ethical clearance from Institutional animal ethical Committee (IAEC). The IAEC number for this study was IAEC-NIMSLAC/18-04. The study was conducted under the Good Laboratory Practices (GLP) environment. The study was performed on male SD rats weighing 200 ± 5 g, housed in polypropylene cages in an air-conditioned room with temperature maintained at 25 ± 2°C and 12 h alternating day and night cycles. The animals were allowed standard rat chow diet and sterile distilled water. Total 30 male SD rats were selected and divided into three groups of ten animals each. These groups are follows:
Group I (n=10): Control group
Group II (n=10): Hemorrhoids induced group (6.0% Croton oil application)
Group III (n=10): Hemorrhoids plus Pilescure treated group (206.66 mg/kg body weight)
Hemorrhoids were induced in the all groups, except control group, by applying the croton oil preparation (pyridine, diethyl ether, deionized water and 6% croton oil in diethyl ether in the ratio of 1:4:5:10 [8]. Followed by overnight fasting animals, sterile cotton swabs (4 mm diameters) soaked with 200 µl of croton oil solution was inserted in to anus for 20-25 s for 7 days. After 7 days, a linear development of edema was observed after croton oil application and recto anal area was measured in all groups by vernier caliper as evidence of inflammation. After changes in the recto-anal area, stool frequency, hematological (WBC, Neutrophil) and C-reactive protein parameters were measured in group II and group III animals, further treatment was started with pilescure drug orally for 21 days only in group III animals. After 21 days treatment, all animals were overnight fasted and 2.0 ml blood samples were collected in heparin and non-heparin vials. The 1.5 ml blood sample was used for serum preparation and measured the oxidative stress (lipid per-oxidation), inflammatory and antioxidant enzymatic parameters and rest 0.5 ml was used for hematlogical examination. After collection of blood samples, animals were euthanized exsanguinations under anesthesia and recto-anal tissue were isolated and weight immediately and fixed in 10% formalin solution for histological examination. The recto-anal coefficient was calculated by using formula.
Recto-anal coefficient=Weight of recto-anal tissue (mg)/Body weight (g)
Hematological examination
Hemoglobin (Hb), White blood cell (WBC), platelet count (PLT), neutrophil were examined through fully automatic cell counter (Mindray Vector, Model No.BC-2300).
Oxidative stress parameter
Free radical mediated damage was assessed by the measurement of lipid peroxidation in the term of malon dialdehyde (MDA) formed, essentially according to method of Ohkawa et al. [20]. It was determined by thiobarbituric reaction. The reaction mixture consisted of 0.1 ml serum samples, 0.20 ml of 8.1% sodium dodecyl sulphate (SDS), 1.5 ml of (20%, pH 3.5) acetic acid, 1.5 ml of 0.8% thiobarbituric acid (TBA) and 0.7 ml distilled water to see find volume of 4.0 ml. The tubes were kept in boiled water at 95°C for 1 h and cooled immediately under running tap water. The amount of 1.0 ml of water was added to and 5.0 ml of the mixture of n-butanol and pyridine (15:1 v/v) and vortexed. The tubes were centrifuged at 3500 rpm for 15-20 min. The upper layer was aspirated and optical density was measured at 532 nm. The molar extension coefficient 1.56 × 105 was used for calculation.
Cytokines assays
Cytokines parameters such as tumor necrosis factor-α (TNF-α), Interlukin-β (IL-β) and Interlukin-6 (IL-6) were assayed in the serum samples by ELISA Reader (Merck, Serial No. 21041098, MIOS-Jounior). The assays were performed according to protocol recommended by the manufacturer’s (Invitrogen San Jose CA, USA).
Nitric oxide assay
The nitrite level was estimated in the serum sample according to method of Tsai et al. [21]. 100 µl of serum sample was mixed with 0.4 ml phosphate buffer saline (0.1 M, pH 7.2) and added 2.0 ml Griess reagent. Then 2.0 ml of 5% TCA solution was added and mixed properly by vortex shaker and kept for incubation for 15-20 min. After incubation, the reaction mixture was centrifuged at 14000x g for 20 min and supernatant was taken carefully in other clean tube and absorbance was recorded at 540 nm. The concentration of nitrite was determined from stranded curve prepared with 0.1 ml of 100 µM sodium nitrite.
Anti-oxidant parameters
We measured the some antioxidant enzymatic parameters viz Catalase [22], reduced glutathione [23], glutathione peroxidase [24] and glutathione reductase [25] by standard methods in the serum samples.
Histopathological analysis
Recto-anal tissue was excised from all groups of animals under anesthesia and fixed in 10% neutralized formalin solution for 24 h, dehydrated in gradual ethanol (50-100%), cleared in xylene and embedded in paraffin wax. The sections, which were 5 mm thick, were then prepared using rotary microtome (Leica RM 2125 RTS, Singapore) and stained with hematoxylin and eosin dye for microscopic observation of histopathological changes using a light microscope.
STATISTICAL ANALYSIS
The results are expressed in mean ± SD. Statistical significance was calculated by one way analysis of variance (ANNOVA) followed by Turkey's t-test multiple comparison test using Graphpad Prism (Version 5, software programe, San Diego, CA, USA). A P
RESULTS
Acute toxicity
In acute toxicity study, the animals did not showed any clinical sign of toxicity and mortality after oral administration of pilescure drug upto 2000 mg/kg dose level, so the LD50 of ayurvedic drug Pilescure was found more than 2000 mg/kg. Hence the approximately 1/10th of minimum dose was selected and administered for the present study.
Effect on recto-anal weight, stool weight and recto-anal coefficient
In the present study, it was examined that a significant (p<0.0001) elevated recto-anal weight and recto-anal coefficient along with (p<0.001) decreased stool weight in hemorrhoid induced group after application of croton oil preparation as compared to control group. After treatment with pilescure drug for 21 days, the recto-anal weight and its coefficient were found statistically significant (p<0.0001) decreased along with improved stool weight in pilescure treated group as compared with hemorrhoids induced group and come back to control group (Table 1).
Effect on hematological and biochemical parameter
It was observed that a statistically (p<0.0001) significant decreased hemoglobin and platelet count levels in the hemorrhoid induced group as compared with control group. After treatment with pilescure for 21 days, these levels were significant (p<0.0001) enhanced in the treated group as compared to induced group and these levels were reached almost near to control group. A white blood cell and C-reactive protein (CRP) levels were also found significant (p<0.0001; p<0.001) increased the in the hemorrhoid induced group as compared with control group. These parameters were significant (p<0.001; p<0.0001, p<0.05) decreased in pilescure treated group after treatment with drug for 21 days (Table 2).
Effect on cytokines parameters
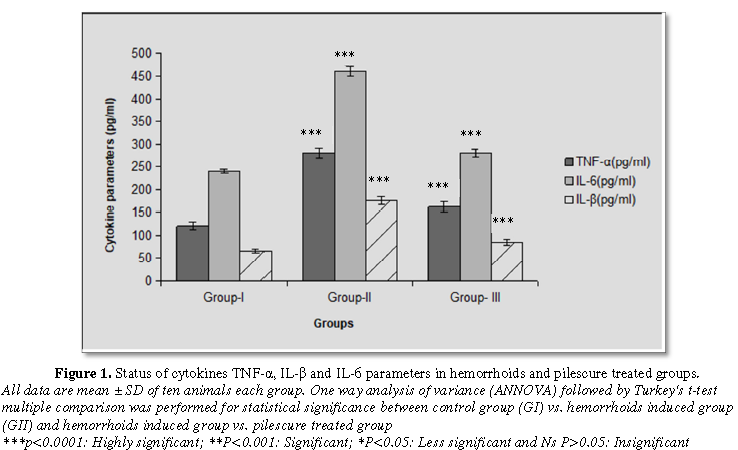
A significant (p<0.0001) enhancement was found in the tumor necrosis factor-α (TNF-α), Interlukin-beta (IL-β) and Interlukin-6 (IL-6) levels in the serum of hemorrhoid induced group after administration of croton oil application. After treatment with pilescure drug for 21 days, these inflammatory parameters were found significantly (p<0.0001) reduced in the treated group and all levels were come back near to control group (Figure 1).
Effect on stress and inflammatory parameter
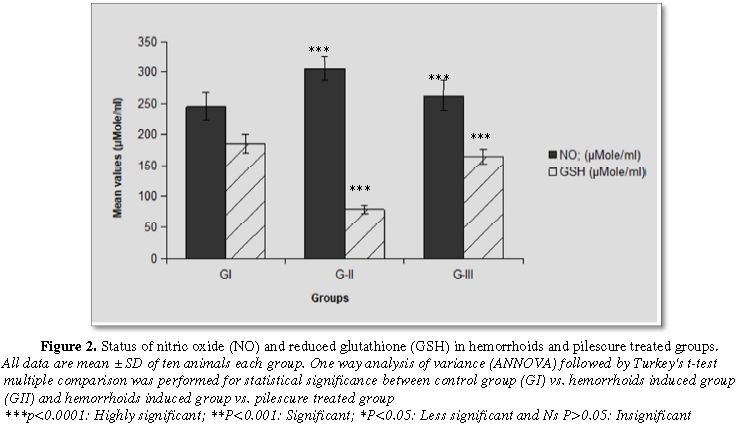
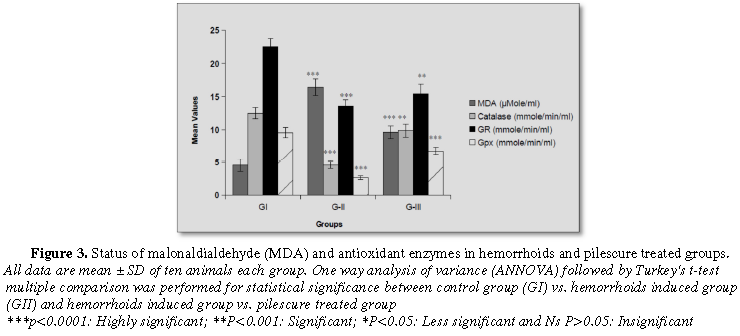
The results revealed that a significant (p<0.0001) enhancement of malonaldialdehyde (MDA) and nitric oxide (NO) levels in hemorrhoid induced group as compared with normal control group. These levels were found statistically significant (p<0.0001) decreased in hemorrhoid plus pilescure treated group after 21 days treatment and come back to control group (Figures 2 and 3).
Effect on antioxidant parameters
A significant (p<0.0001; p<0.001) reduction of antioxidant enzymes viz. catalase, glutathione peroxidase, glutathione reductase and reduced glutathione enzyme activities were found in hemorrhoid induced group as compared with control group. After treatment with pilescure drug in hemorrhoid plus pilescure treated group, these enzymes activities were significantly (p<0.0001; p<0.001) increased when compared with hemorrhoids induced group. These enzyme activities were normalized and come back to control group (Figures 2 and 3).
Histopathological examination
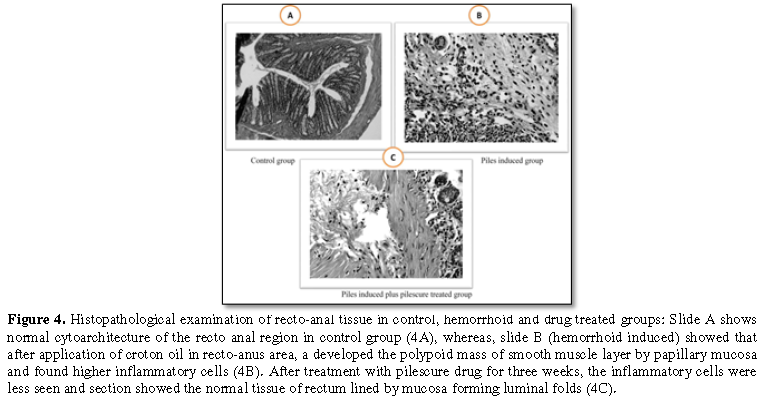
The histological examination of recto-anal tissue showed that partly skin and anal mucosa with normal cytoarchitecture of the recto-anal region found in control group (Figure 4A) whereas group II treated with croton oil application developed the polypoid mass of smooth muscle layer by papillary mucosa and significantly found higher inflammatory cells in recto-anal region (Figure 4B). After treatment with pilescure drug in treated group, the section showed that the inflammatory cells were seen less and tissue of rectum lined found by mucosa forming luminal folds (Figure 4C).
normal cytoarchitecture of the recto anal region in control group (4A), whereas, slide B (hemorrhoid induced) showed that after application of croton oil in recto-anus area, a developed the polypoid mass of smooth muscle layer by papillary mucosa and found higher inflammatory cells (4B). After treatment with pilescure drug for three weeks, the inflammatory cells were less seen and section showed the normal tissue of rectum lined by mucosa forming luminal folds (4C).
DISCUSSION
Hemorrhoids are a pathological state that is characterized by a severe vasodilatation at the region of recto-anal area that causes the inflammation surrounding tissues, thus further leading to secondary complications such as extravasation of fluid into fluid interstitial space mainly due to increased vascular permeability and migration of large amount of inflammatory cells [8]. So in this study croton oil used as inducer for induction of hemorrhoid model in animals. Croton oil causes inflammation due to release of soluble factors inflammatory lipid metabolites such as prostaglandins [26], leukotrienes, nitric oxide (NO) [27] and cytokines (TNF-α, IL-6 and IL-β) [28,29], etc. The present finding suggested that a significant improvement in stool frequency on the basis of stool weight examination in pilescure treated group when compared with hemorrhoid induced group. The result clear indicated that drug suppresses the constipation symptom which is one of the causative factors for hemorrhoid disease. Such action was seemed due to presence of active ingredients Terminalia chebula in a drug which act as laxative and vata detoxification and digestive balancing. Recto-anal weight and recto-anal coefficients were found significantly decreased along with increased the hematological parameters after treatment with drug for 21 days in pilescure treated group as compared with hemorrhoid induced group. After treatment with drug these parameters were come back to control group. These findings clear suggesting that the active ingredients of drug shows potent anti-inflammatory effect and improve the hematological parameter due to homeostatic balance and its main action on the blood capillaries, due to its KASHAYA RAS (Astringent) and SHEET VIRYA (cool nature). The results also showed that there were significantly increased inflammatory parameters C-reactive protein, neutrophil, cytokines (TNF-α, IL-6 and IL-β) and nitric oxide in hemorrhoid induced group when compared with control group. These results are indicating that croton oil leads to inflammation due to release of soluble factors involving inflammatory lipid metabolites, kinins (chemokines) and cytokines, etc. These factors alone or and/or in combination, regulate the activation of resident cells (fibroblast, microphages, endothelial cells and mast cells) and newly recruited inflammatory cells (lymphocytes, basophil and neutrophil) causes the systemic response to inflammation [30]. After treatment with drug for 21 days, these inflammatory parameters were significantly decreased in pilescure treated group as compared with hemorrhoid induced group and come near to control group. The inflammatory parameters were reduced in pilescure treated group due to presence of anti-inflammatory property of Terminalia chebula, Mesua ferrea and Ricinus communis herbs present in the drug. Various studies have been reported that these herbs shows as anti-inflammatory effect [31-34].
Oxidative damage to biological compounds, especially lipids through lipid peroxidation has been shown to play a significant role in various pathophysiological states. Superoxide dismutase (SOD) is a specific antioxidant enzyme that repairs the cells and reduced the damage caused by superoxide anions (O-2), which is most common free radical in the body. Free radical can lead to lipid peroxidation in organisms. Malonaldialdehyde (MDA) is end product of poly unsaturated fatty acids (PUFAs) in cells, and an increase in free radical causes overproduction of MDA. MDA is biomarker of oxidative stress in various diseases [35]. So authors have measured the malonaldialdehyde level as a biomarker of oxidative stress. In normal cell, there is proper balance between oxidative stress and antioxidant enzyme levels, any imbalance between these two ratios that lead to various pathophysiological states. In our study, we found that there were statistically (p<0.0001) significant decreased antioxidant enzymes activities (Catalase, glutathione peroxidase, glutathione reductase and reduced glutathione) along with increased MDA level in hemorrhoid induced group as compared with control group. After treatment with pilescure drug for 21 days, these enzymatic activities were significant improved along with lowered the MDA level in pilescure treated group when compared with hemorrhoid induced group. These results clear demonstrated that the drug consists of polyherbal has prominent antioxidant effect. The similar result has been published by other investigator in croton oil hemorrhoid induced animals [10]. Based on above findings it is clear indicating that croton oil application causes severe inflammation that altered recto-anal physiology, hematological, biochemical and cytokines parameters along with enhancement of oxidative stress that leads to hemorrhoid. After treatment with pilescure drug the above parameters were improved in hemorrhoid condition. Base on above finding it is clear indicating that the poly herbal drug pilescure is most therapeutic effects for reduction of recto-anal pain, inflammation and inhibit the free radical generation and enhance the antioxidant enzymes level. These anti-inflammatory and antioxidant properties are present in a drug due to presence of active constituents of tannin, alkaloids, and polyphenol ingredients in polyherbal ayurvedic formulation.
CONCLUSION
So conclusion these finding suggesting that pilescure is nontoxic with LD50 more than 2000 mg/kg body weight and it is most effective ayurvedic polyherbal drug which reduces inflammation, oxidative stress and antioxidants properties along with improvement of haematological parameters during hemorrhoid condition. The drug can improve the healthy and quality of life people who are suffering from hemorrhoid problem.
ACKNOWLEDGEMENT
The authors are thankful to Mr. Harish Yadav (Technical officer) to provide the histopathological results. Authors are also thankful to sponsor, formulation department of Lavanya Ayurvedic Pvt. Ltd., Lucknow to provide test item pilescure for carried out the study.
COMPETING INTERESTS
None
SOURCE OF SUPPORT
None
1. Lohsiriwat V (2012) Hemorrhoids: From basic pathophysiology to clinical management. World J Gastroenterol 18: 2009-2017.
2. Kaidar PO, Person B, Wexner SD (2007) Hemorrhoidal disease: A comprehensive review. J Am Coll Surg 204: 102-117.
3. Chong PS, Bartolo DCC (2008) Hemorrhoids and fissure in ano. Gastro Clin Nort Am 37: 627-644.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD (2009) What every gastroenterologist needs to know about common anorectal disorders? Word J Gastroenterol 15: 3201-3209.
5. Lorengo RS (2009) Hemorrhoids: Diagnosis and current management. Am Surg 75: 635-642.
6. Rattan S (2005) The internal and sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17: 50-59.
7. Mohamed FA, Badraddin MA, Kamal-Eldin HET (2013) Some pharmacological action of Myrica rubra Part 1: Effect on experimentally-induced gastric ulcers, inflammation in hemorrhoids in rats. Afr J Pharm Pharmacol 7: 512-516.
8. Mohammed A, Gollapalle LV, Mohamed R, Agadi HMT, Mirza RB, et al. (2014) An improved experimental model of hemorrhoids in rats: Evaluation of anti-hemorrhoidal activity of an herbal formulation. ISRN Pharmacol 7: 1-7.
9. Plapler H (2006) Hemorrhoids: An experimental model in monkeys. Acta Cir Bras 21: 354-356.
10. Ustunova S, Ergin B, Gurel E, Tan N, Caner M, et al. (2013) Herbal hemorrhoidal cream for hemorrhoids. Chin J Physiol 56: 253-262.
11. Nishiki K, Nishinaga K, Kudoh D, Iwai K (1988) Croton oil-induced hemorrhoid model in rat: Comparison of anti-inflammatory activity of diflucortolone valerate with other glucocorticoids. Nihon Yakurigaku Zasshi 92: 215-225.
12. Lohsiriwat V (2012) Hemorrhoids: From basic pathophysiology to clinical management. World J Gastroentrol 18: 2009-2017.
13. Lohsiriwat V (2015) Treatment of haemorrhoids: A coloproctologist's veiw. World J Gastroentrol 21: 9245-9252.
14. Mohamed SIJ, Zakia S, Md. Ershad A, Mst. Marium B, Md. Mominul H (2014) Evaluation of analgesic and anti-inflammatory activities on ethanolic extract of Terminalia chebula fruits in experimental animal models. Am J Plant Sci 5: 63-69.
15. Gopalakrishnan C, Shankaranarayanan D, Nazimudeen SK, Viswanathan S, Kameswaran L (1980) Anti-inflammatory and CNS depressant activities of xanthones from Calophyllum inophyllum and Mesua ferrea. Indian J Pharmacol 12: 181-191.
16. Ilavarasan R, Mallika M, Venkataraman S (2006) Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol 103: 478-480.
17. Dnyaneshwar JT, Maruti GW, Rajendra SB, Ravindra YP (2011) Antinociceptive activity of Ricinus communis L. leaves. Asian Pac J Trop Biomed 1: 139-141.
18. Pampattiwar SP, Adwani NV, Bulusu S, Paramkusha RM (2013) Pharmacological study of anti-inflammatory action of Haritaki preparation on wistar rat in hemorrhoids. Global J Med Res Plants Indigen Med 2: 178-182.
19. Parasuraman S (2011) Toxicological screening. J Pharmacol Pharmacother 2: 74-79.
20. Ohkawa H, Ohishi N, Yagi K (16979) Assay of lipid peroxidation in animal tissue by thiobarbutric acid reaction. Anal Biochem 95: 351-358.
21. Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, et al. (1997) Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun 65: 1870-1875.
22. Aebi H (1984) Catalase. In: Packer L, editor. Methods in Enzymol. Orlando, FL: Academic Press, pp: 125-126.
23. Ellman GL (1959) Tissue sulfhydrl groups. Arch Biochem Biophys 82: 70-77.
24. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, et al. (1973) Biochemical role as a component of glutathione peroxidase. Science 179: 588-590.
25. Carlberg I, Mannervik B (1985) Glutathione reductase. Meth Enzymol 113: 485-490.
26. Bhukya B, Anreddy RN, William CM, Gottumukkala KM (2009) Analgesic and anti-inflammatory activity of leaf extract of Kydia calcina Roxb. Bangladesh J Pharmacol 4: 101-104.
27. Bilici D, Akpinar E, Tunc AK (2002) Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol Res 46: 133-190.
28. Bhavin VA, Ruchi VB, Shrikant JV, Dev SD (2010) Evaluation of anti-inflammatory activity of n-butanol extract from Pergularia daemia in experimental animal model. J Pharm Res 3: 3039-3044.
29. Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, et al. (2007) Cytokine profiles during Carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 8: 127-136.
30. Carol AF, Timothy MW (1997) Cytokines in acute and chronic inflammation. Front Biosci 2: 12-26.
31. Mohamed SIJ, Zakia S, Md. Ershad A, Mst. Marium B, Md. Mominul H (2014) Evaluation of analgesic and anti-inflammatory activities on ethanolic extract of Terminalia chebula fruits in experimental animal models. Am J Plant Sci 5: 63-69.
32. Gopalakrishnan C, Shankaranarayanan D, Nazimudeen SK, Viswanathan S, Kameswaran L (1980) Anti-inflammatory and CNS depressant activities of xanthones from Calophyllum inophyllum and Mesua ferrea. Indian J Pharmacol 12: 181-191.
33. Ilavarasan R, Mallika M, Venkataraman S (2006) Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol 103: 478-480.
34. Dnyaneshwar JT, Maruti GW, Rajendra SB, Ravindra YP (2011) Antinociceptive activity of Ricinus communis L. leaves. Asian Pac J Trop Biomed, pp: 139-141.
35. Kulkarni SK (1999) Handbook of Experiential Pharmacology. 2nd Edn. Delhi: Vallabh, Parkashan, pp: 112-113.