922
Views & Citations10
Likes & Shares
Drug-induced gingival
hyperplasia is an abnormal enlargement of the gingival tissues, which can occur
as a side effect of Cyclosporine A, following renal transplantation. It is a
debilitating condition, which can result in increased dental infection rates,
tooth loss and subsequent reduced masticatory function. Although
immunosuppressive agents such as Cyclosporine A are known to cause this oral
side effect, many other medications (such as Nifedipine) and anticonvulsant
(such as Phenytoin) which are not uncommonly used in renal transplant patients can
exacerbate this condition. With the improving survival rates of renal
transplant patients, more patients are on long-term immunosuppressive regimens
and therefore we see an increase in the prevalence of gingival hyperplasia in
the population. The aim of this article is to present the aetiology,
pathogenesis and management of gingival hyperplasia.
INTRODUCTION
CLINICAL MANIFESTATIONS
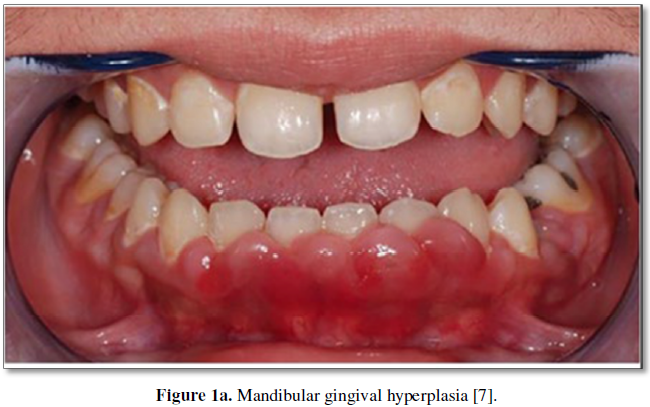
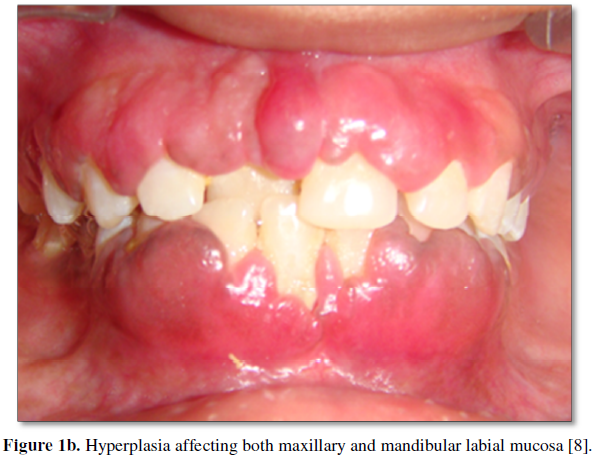
Drug-induced gingival overgrowth commonly presents within 3 months of commencing Cyclosporine treatment as enlargement of the interdental papilla [4,5]. It can extend to involve the remainder of the gingival tissues, extending apically past the mucogingival margin or coronally towards the occlusal surface. It is more commonly seen in the anterior mandible, followed by the anterior maxilla (Figures 1a and 1b) [6]. The tissues tend to have an erythematous appearance; however, the degree of cellularity, inflammation and fibrosis have been shown to vary according to factors such as genetics and oral hygiene [4], all of which will be discussed later in the paper.
The pathogenesis
underlying drug-induced gingival overgrowth is not fully understood. However,
it is most likely multifactorial, with a plethora of contributing hormones and
cell mediators. Studies have shown that CsA, along with anti-hypertensives and
anticonvulsants alter the metabolism of gingival fibroblasts, reducing the
activity of lysosomal enzymes, and decreasing the phagocytosis of the gingival
fibroblasts [9,10]. As a result, the apoptosis and turnover of the fibroblasts
are reduced, resulting in accumulation within the gingivae. It has also been
suggested that there is an overall reduction of collagenase gene expression,
further reducing the degradation of gingival cells [10]. Other studies have
demonstrated an increase in the prevalence of chemical mediators such as
angiotensin II, endothelin-1, transforming growth factor (TGF-b), connective tissue growth factor (CTGF) as well as an insulin-like
growth factor (IGF). All of these hormones resulted in the activation of
gingival fibroblasts and increased fibrous tissue deposition [4]; their
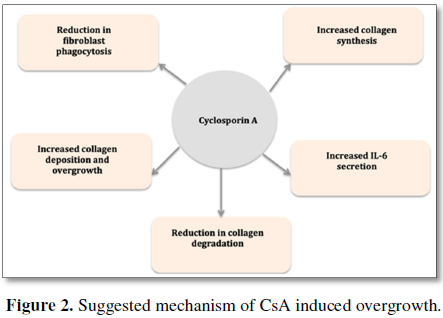
specific mechanisms are summarized in Figure
2. In all the overall conclusion of these studies is that the presence of
CsA promotes fibroblast formation and reduces matrix metalloproteinase
production and thus fibroblast degradation (Figure
2).
RISK FACTORS
There are many factors that have been suggested to influence an individual’s susceptibility to the development and severity of gingival hyperplasia. Medication dose is a controversial variable, with opinions divided regarding whether it does; in fact, affect the onset and severity [3]. Animal studies showed a significant relationship between the dose of Nifedipine and the severity of the hyperplasia; however, when combined with Cyclosporine dose showed no significant impact [1]. Although there is no clear answer to this, it is generally accepted that in order for gingival overgrowth to develop, a threshold serum concentration of causative agent needs to be reached [12]. Studies have gone on to show that gender and sex may have a part to play in susceptibility, with a reported genetic predisposition associated with HLA DR-1, HLA DR-2 and HLA-B37 genes [13]. Furthermore, adolescent females are thought to be more susceptible due to the production of a sex hormone that produces a metabolite that results in increased collagen synthesis [14].
A proven risk factor is oral hygiene. Not only does the
presence of plaque initiate a local inflammatory response that results in
gingival erythema and edema, but these inflammatory changes have been shown to
increase the interaction between CsA and fibroblasts, resulting in
proliferation4. Plaque control is paramount in reducing the severity of the
overgrowth, as maintaining clean gingival tissues has been shown to reduce the
overgrowth by 40% [11]. Furthermore, the presence of hyperplastic gingival
tissues acts as a plaque trap and encourages bacteria accumulation, therefore
patients with poor oral hygiene are not only more susceptible to developing
gingival hyperplasia, but they more likely to have further increases in inflammation
and overgrowth over time [3]. A longitudinal study measuring the severity of
CsA induced gingival hyperplasia showed that the hyperplasia was less severe in
patients with a given oral hygiene regime in comparison to those without [3].
MANAGEMENT
Non-surgical
The use of non-surgical management options should be used as
first-line treatment for patients with gingival overgrowth. Not only are they
the most conservative options and therefore have fewer associated risks, but
they can increase the success of future surgical therapies. Ideally the most
effective non-surgical management modality would be ceasing the use of CsA,
however understandably this is not an option, as it would result in a drastic
increase in allograft rejections following transplantation. When possible, the
use of Tacrolimus as an alternative to CsA would be advised, as it has been
shown to have fewer oral side effects, with only 30% of patients presenting
with gingival hyperplasia [5] in comparison to the up to 70% prevalence seen with
Cyclosporine [2]. This medication change may not be suitable for all patients,
and therefore other non-surgical methods may need to be considered.
Due to the strong relationship between severity and oral
hygiene all patients should have a thorough oral hygiene regime regardless of
any other non-surgical or surgical treatment options. This regime is usually
best made with the input of a dental surgeon and a dental hygienist, in order
to ensure that the patient is receiving the most appropriate advice tailored to
them. This regime may include regular hygienist appointments for scaling or
root surface debridement [15-17]. The liaison with a dental practitioner allows
the provision of multidisciplinary care and ensures that the gingival
hyperplasia is being closely monitored.
The use of topical antifungal agents such as Nystatin on the
enlarged interdental papilla of immunosuppressed patients has been reported to
aid resolution the lesions [18]. Clinical trials have also suggested the use of
systemic Azithromycin to help reduce the lesions as well as the use of
non-steroidal inflammatory agents to reduce the IL-1 mediated inflammation
[19]. Although these options are not suggested as being first-line options in
the literature, it may be worth considering them as adjunctive treatment
modalities.
Surgical
Non-surgical treatment may only reduce the severity of the
hyperplastic gingiva, and therefore consideration of surgical options may be
needed. When periodontal surgery is to be considered, a discussion with a restorative
dental surgeon within secondary care is necessary to determine the appropriate
surgical approach. Many surgical options are available, including flap surgery,
gingivectomy, laser debulking and electrosurgery [5]. Consideration for the
underlying bone anatomy (presence of osseous defects) the presence of active
periodontitis and the gingival biotype is vital [5]. Although surgical removal
of the hyperplastic tissues initially provides good outcomes, recurrence was
observed in 34% of surgical cases within 18 months. Other studies have even
shown recurrence within 3-6 months of surgery [5]. These factors need to be
considered before embarking on surgical treatment as the unpredictable
long-term improvement seen may not be worth the surgical risk and the painful
post-operative recovery.
Other immunosuppressive
medications and gingival hyperplasia
Although CsA is the medication that is most commonly
associated with gingival hyperplasia in renal transplant patients, there are
other medications that may be used that also exacerbate this condition.
Tacrolimus is commonly used as a replacement therapy in cases where CsA has
caused severe gingival hyperplasia as is known to have fewer oral side effects;
however it is not devoid of risks. Patients receiving Tacrolimus still present
with gingival overgrowth, however it is to a lesser extent (only 30% of
patients) [5]. Other potent immunosuppressive medications such as the mTOR
inhibitor sirolimus has been shown to also result in gingival hyperplasia, but
again to a lesser extent in comparison to CsA. A study comparing the presence
of gingival overgrowth in patients on different immunosuppressive regimens
revealed that CsA resulted in hyperplasia in 59.1% of the sampled patients in
comparison to 12.0% and 16.7% for patients treated with tacrolimus and
sirolimus, respectively [20].
Interestingly anti-metabolites such as Azathioprine and
Mycophenolate Mofetil (MMF) have been shown to have a protective effect, with
reduced prevalence of gingival hyperplasia in patients on combination therapies
rather than CsA alone. In this study a combination of CsA and Azathioprine
resulted in 60.2% of cases presenting with hyperplasia and a combination of CsA
and MMF resulted in 47.2% prevalence [20]. When comparing this to the 100%
prevalence of gingival hyperplasia in patients receiving CsA and prednisolone
not only does this indicate that Azathioprine and MMF have a protective
function on the gingiva, but it indicates that prednisolone can exacerbate the
effect of CsA [20]. The respective effects of these drugs on the gingiva can be
seen in Table 2.
CONCLUSION
Although drug-induced gingival hyperplasia in renal transplant
patients may be unavoidable in some cases, there are non-surgical treatment
modalities that can be adopted to allow patients to manage this condition.
Although the histopathology and etiology are not fully understood, clinicians
can agree on the importance of meticulous oral hygiene for patients. Patients
should be warned of the risks of CsA and Nifedipine, as this will allow them to
take the required precautions to help reduce their risk. Consideration for
combination therapy using Azathioprine and MMF may help reduce the prevalence
of gingival hyperplasia in transplant patients. Multi-disciplinary management
of these patients is imperative in ensuring holistic patient care.
1.
Khoori AH, Einollahi A, Ansari B, Moozeh MB (2003) The effect of cyclosporine with and without
nifedipine on gingival overgrowth in renal transplant patients. J Can Dent Assoc 69: 236-241.
2.
Aral CA, Dilber E, Aral K, Sarica Y, Sivrikoz ON (2015) Management of cyclosporine and nifedipine-induced
gingival hyperplasia. J Clin Diagn Res 9: 12-15.
3.
McCaughan G
(2004) Molecular Approaches to the Side effects of immunosuppressive drugs.
Transplantation 78: 1114-1115.
4.
Subramani T, Rathnavelu V, Alitheen NB (2013) The possible potential therapeutic targets for
drug induced gingival overgrowth. Mediators Inflamm 2013: 639468.
5.
Ciavarella D, Guiglia R, Campisi G, Di Cosola M, Di
Liberto C, et al. (2007) Update on
gingival overgrowth by cyclosporine A in renal transplants. Med Oral Patol Oral
Cir Bucal 12: E19-25.
6.
Ghafari A, Poorabbas R, Takieh JA, Sepehrvand N, Kargar
C, et al. (2010) Gingival enlargement and its risk factors in
kidney transplant patients receiving cyclosporine A. Iran J Kidney Dis 4: 66-70.
7.
Ponnaiyan D, Jegadeesan V (2015) Cyclosporine A: Novel concepts in its role in drug-induced gingival overgrowth. Dent Res J (Isfahan) 12: 499-506.
8.
Dental Health Tips (2019) Gingival hyperplasia causes and complications in
children [online]. Available from: https://www.dentaltipsforall.com/tag/gingival-hyperplasia-ppt/
9.
Nappalli D, Lingappa A (2015) Oral manifestations in transplant patients. Dent Res J
(Isfahan) 12: 199-208.
10.
Kim JY, Park SH, Cho KS, Kim HJ, Lee CK, et al. (2008) Mechanism of azithromycin treatment on gingival
overgrowth. J Dent Res 87:
1075-1079.
11.
Trackman PC, Kantarci A (2015) Molecular and clinical aspects of drug-induced gingival
overgrowth. J Dent Res 94: 540-546.
12.
Somacarrera ML, Hernandez G (1994) Factors related to the incidence and severity of
cyclosporine-induced gingival overgrowth in transplant patients: A longitudinal
study. J Periodontol 65:
671-675.
13.
Seymour RA, Thomason JM (1996) The pathogenesis of drug-induced gingival overgrowth. J
Clin Periodontol 23: 165-175.
14.
Sooriyamoorthy M, Gower DB (1990) Androgen metabolism in gingival hyperplasia induced by
nifedipine and cyclosporine. J Periodontal Res 25: 25-30.
15.
Mavrogiannis M, Ellis JS, Thomason JM, Seymour RA (2006) The management of
drug-induced gingival overgrowth. J Clin Periodontal 33: 434-439.
16.
Dhale RP, Phadnaik MB (2010) Conservative management of amlodipine induced gingival
enlargement. J Indian Soc Periodontal 14: 279-281.
17.
Ilgenli T, Atilla G, Baylas H (1990) Effectiveness of periodontal therapy in aptients
with drug-induced gingival overgrowth. Long-term results. J Periodontol 70: 967-972.
18.
Dongari-Baqtzoglou V, Reseatch, Sciene and Therapy Committee; American Academy of Periodontology (2004) Drug-associated gingival enlargement. J Periodontal 75: 1424-1431.
19.
Lu HK, Tseng CC, Lee YH, Li CL, Wang LF (2010)
Flutamide
inhibits nifedipine- and interleukin-1-beta induced collagen overproduction in
gingival fibroblasts. J Periodontal Res 45: 451-457.
20.
de la Rosa Gracia E, Mondragon Padilla A (2009) The effect of mycophenolate mophetil and
azathioprine on gingival overgrowth associated with cyclosporine A use in
kidney transplant patients. Nephrologia 29: 1-502.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Alcoholism Clinical Research
- Dermatology Clinics and Research (ISSN:2380-5609)