1494
Views & Citations494
Likes & Shares
Peripheral arthralgia is a common complaint of peri- and post-menopausal women. Although the pathophysiology is not completely understood, it is thought that menopausal arthralgia may be related to estrogen deficiency and depletion. Menopausal women referred to rheumatologists with these complaints along with high titer anti-nuclear antibody (ANA) can lead to patient anxiety and present a diagnostic dilemma for the clinician. We report two index cases of menopausal women who had a positive ANA by indirect immunofluorescence (IIF) attributed to anti-dense fine speckled 70 (DFS70) autoantibody (Aab) that had developed arthralgias prompting referral to our clinic. It is known that the presence of this Aab is paradoxically found more frequently in healthy individuals compared to those with a systemic autoimmune rheumatic disease (SARD). The lack of evidence for a SARD in these patients and a remarkable response to hormone replacement therapy (HRT) prompted a wider study of anti-DFS70 Aab in peri- and post-menopausal women. Hence, a retrospective analysis of 282 Japanese female patients referred to an outpatient rheumatology clinic for arthralgias was performed. The results indicate that the frequency of anti-DFS70 Aab in peri- and post-menopausal women with non-SARD is higher than in other peri- and post-menopausal women with SARD. Therefore, testing for anti-DFS70 Aab in this population may be helpful in distinguishing between SARD and non-SARD patients with positive ANA, who may in fact, have menopausal-related arthralgia and are responsive to HRT.
Keywords: Antinuclear antibody (ANA), Anti-DFS70/LEDGF, Estrogen deficiency hormone replacement therapy, Menopausal arthralgia
Abbreviations: AAB: Autoantibody; ACR: American College of Rheumatology; ANA: Anti-Nuclear Antibody; DFS: Dense Fine Speckled; ELISA: Enzyme-Linked Immunoassay; EULAR: European League Against Rheumatism; FM: Fibromyalgia; FSH: Follicle Stimulating Hormone; HIV: Human Immunodeficiency Virus; HRT: Hormone Replacement Therapy; ICAP: International Consensus on ANA Patterns; IIF: Indirect Immunofluorescence; HTLV 1: Human T-Cell Leukemia Virus 1; mg: milligram; NASH: Non-Alcoholic Steatohepatitis; OD: Optical Density; OA: Osteoarthritis; PCNA: Proliferating Cell Nuclear Antigen; PeMA: Peri-Menopausal Arthralgia, PeMS: Peri-Menopausal Stage; PMS: Pre-Menopausal Syndrome; PoMA: Post-Menopausal Arthralgia, PoMS: Post-Menopausal Stage; PrMA; Pre-Menopausal Arthralgia, PrMS: Pre-Menopausal Stage; RA: Rheumatoid Arthritis; SARD: Systemic Autoimmune Disease; SjS: Sjögren’s Syndrome; SLE: Systemic Lupus Erythematosus; SSc: Systemic Sclerosis; UA: Undifferentiated Arthralgia/Arthritis
INTRODUCTION
Peripheral joint arthralgia along with menopausal symptoms of palpitations, hot flashes and diaphoresis are common complaints of peri- and post-menopausal women [1]. The cause of these musculoskeletal symptoms in this clinical setting is not completely established, although circumstantial evidence suggests it is associated with estrogen deficiency. In younger females, arthralgias often appear during the premenstrual stage as a part of premenstrual syndrome. Even in the peri-menstrual stage, menses become irregular because of fluctuating estrogen levels and the antioxidant α-tocopherol-N that is required to maintain regular periods [2]. In the post-menopausal stage, joint pains can be relieved by hormone replacement therapy (HRT) [3].
The clinical picture of arthralgia associated with menopause can become clinically challenging if a screening anti-nuclear antibody (ANA) by indirect immunofluorescence (IIF) test done to rule out a SARD is found to be positive. We present two index cases of menopausal females with arthralgia and high titer ANA. The ANA described in the sera of these two patients was characteristic of the dense fine speckled (DFS) pattern in nuclei of interphase cells and also of the metaphase plate of dividing cells (International Consensus on ANA Patterns IC-2: https://anapatterns.org/view_pattern.php?pattern=20) and on further testing the autoantibody (Aab) reactivity was confirmed to be directed against the 70 kDa DFS70 target autoantigen [4,5], also known as lens epithelium-derived growth factor (LEDGF) [5,6]. In general, this antibody is found in the sera of less than 10% of SARD patients but paradoxically found in in 10-25% of healthy subjects [4,5,7,8]. On rheumatological evaluation, no evidence of SARD was found in these two patients and after two weeks of HRT, their arthralgias had completely resolved.
While anti-DFS70 Aab has been reported in wide range of diseases and healthy cohorts [4,5,7,8], no studies have focused on its presence in menopausal females. These two case reports of menopausal women with anti-DFS70 Aab without SARD prompted us to determine the frequency of anti-DFS70 Aab in pre-, peri- and post-menopausal females with and without SARD. Since the frequency of anti-DFS70 has been reported to decrease with age [9], but is higher in pre-pubertal females [10] a working hypothesis is that it should also have higher frequency in post-menopausal than in pre- or peri-menopausal females. Further, we set out to determine the frequency of anti-DFS70 Aab in females with undifferentiated arthritis (UA) as compared to patients with a defined SARD.
Patient 1
A 41 year old Japanese female was evaluated for recurrent chest discomfort and back pain. There was no evidence of pathology on chest X-ray or CT scan. Due to concerns of an underlying SARD, an ANA by IIF was done and was found to be positive at a titer of 1:320 with a homogenous and speckled pattern. She had no other manifestations of a SARD. Six years later, her menopausal status was established and within 7 months she could not hold a pencil or pick up coins owing to finger and wrist joint pain. She was seen at a rheumatology clinic for an evaluation of possible rheumatoid arthritis (RA). The laboratory and clinical features are summarized in Supplemental Figure 1a which included a negative autoantibody profile except for a strongly positive anti-DFS70 Ab (OD index 147.1 units). A diagnosis of RA or other related-SARD was ruled out by the attending rheumatologist. After 2 months of HRT, her arthralgias completely resolved.
Patient 2
A 27 year old Japanese woman was noted to have a positive ANA by IIF at a titer of 1:160 with a homogenous and specked pattern and after initial evaluation the diagnosis of SARD was ruled out. At the age of 52, her menses became irregular and within 5 months, she noticed joint pains localized to interphalangeal finger and bilateral knee joints. She was referred to a rheumatology clinic to evaluate the joint pains and laboratory and clinical assessment were unremarkable (Supplemental Figure 1b). In this peri-menopausal stage, she was treated with α-tocopherol N (600 mg/day) and non-steroidal anti-inflammatory medications which, within 2 months, gave her a 50% reduction of joint pain. However, as she entered full menopausal stage, she left her job as an office employee because of severe fatigue and anxiety. Within 2 weeks of cyclic HRT, she became asymptomatic and returned to her employment. Her follow-up ANA by IIF was positive at 1:80 with homogenous and speckled IIF pattern and further evaluation disclosed a positive anti-DFS70 antibody (OD index 80.9) test.
METHODS
Patient population
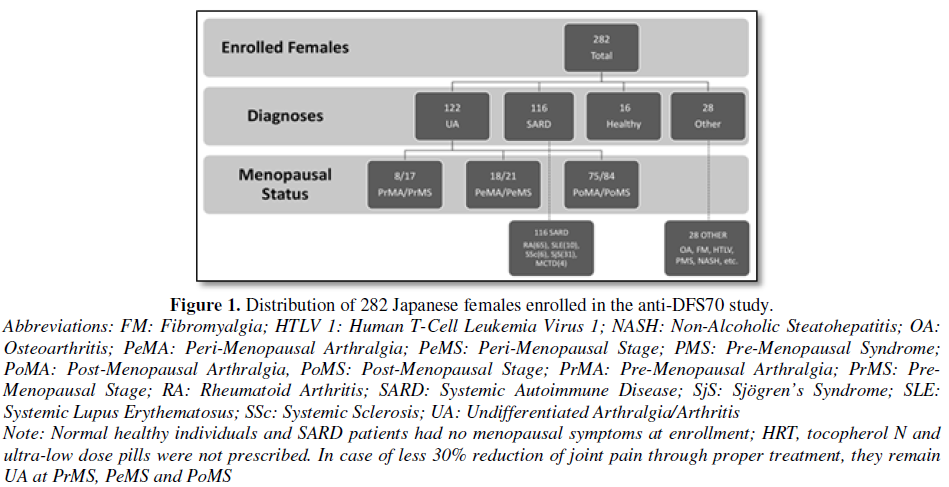
Two hundred and eighty-two female patients seen for evaluation of joint pain at the Keigu and Higashiterao Second Clinics (Yokohama, Japan) from November 2016 to October 2017 were enrolled and separated into 3 main clinical diagnostic groups: SARD, UA and non-SARD (e.g. Osteoarthritis (OA), Fibromyalgia (FM), healthy individuals (HI)) (Figure 1). A diagnosis of an SARD including RA [11], Sjögren’s Syndrome (SjS) [12], Systemic Lupus Erythematosus (SLE) [13] or other SARDs was confirmed by a rheumatologist using appropriate classification criteria. Patients who did not meet full classification criteria for a SARD were classified as UA [14]. Patients with other non-SARD diseases such as OA, FM and healthy individuals were also included as controls. Ethical approval of this study was provided by The Society of Healthcare for Menopause and Aging in Tokyo, Japan and was compliant with the Helsinki Declaration of 1975 as revised in 2013:
Based on the regularity of menses, the UA patients were divided into three categories: Pre-Menopausal (PrM), Peri-Menopausal (PeM) and Post-Menopausal (PoM) stages. These patients were further categorized based on the presence of joint pain as Pre-Menopausal Arthralgia (PrMA), Peri-Menopausal Arthralgia (PeMA) and Post-Menopausal Arthralgia (PoMA) [15]. The definition of menopause included cessation of menses for at least 1 year, follicle stimulating hormone (FSH) levels of ≧ 40 mIU/ml and <20 pg/ml of estradiol [16]. Each patient’s age and baseline joint pain score (visual analog scale: VAS 100 mm in length anchored by 2 verbal descriptors [17]) was documented on the first visit and at subsequent visits. Any reduction of VAS was noted. FSH and estrogen levels were determined on the first visit, then after two months and semi-annually thereafter.
Hormone replacement therapy
Premenopausal women were treated with ultra-low dose norethisterone (1 mg/ethynylestradiol 0.035 mg), PeMA women with α-tocopherol N 600 mg and post-menopausal women with HRT for at least two months, respectively. For women less than 45 or over 60 and those with contraindications to HRT, HRT was not used. The same therapy was continued in patients who experienced a good response. Estrogen monotherapy (i.e., estrogen replacement therapy (ERT)) was administered to PoMA women with a previous hysterectomy. In females without a hysterectomy, monthly treatment with a 17β-Estradiol patch (0.72 mg)/2 days for 26 days and dydrogesterone (duphaston) 10 mg for 10 days, followed by 5 days off the treatment was administered. In PoMA patients ≧ 5 years, estradiol 1mg/levonorgestrel 0.04 mg) 1 tablet/day was prescribed as EPRT continuous. In patients ≧ 60 years of age, estriol (1 mg) 2 tablets/day was prescribed and dydrogesterone 10 mg × 10 days were taken every 6 months.
ANA and anti-DFS70 autoantibody determination
Serum ANA was detected by IIF on HEp-2 cells (Medical and Biological Laboratories Co. Ltd. (MBL), Nagoya, Aichi, Japan). A detectable cellular staining pattern at a serum dilution of ³ 1:40 was regarded as positive. Anti-DFS70 Aab was measured by enzyme-linked immunosorbent assay (ELISA, MBL) and the cutoff of 15 units was established according to the manufacturer’s instructions.
Statistical methods
Statistical analysis included the frequency of anti-DFS70 in all SARD and non-SARD PrMA vs. PeMA vs. PoMA women; the frequency of anti-DFS70 in SARD vs. non-SARD PeMA women; the correlation between ANA IIF and anti-DFS70 Aab. The Mann-Whitney U test and χ2-tests were used for clinical significance.
RESULTS
Baseline demographics and serological profile
The menopausal status and diagnostic distribution of the 282 Japanese females enrolled in this study are shown in Figure 1. Their age range was 29-91 years (median=58). 122 had UA, 116 had a SARD, 28 had ‘other’ diagnoses and 16 were healthy individuals. Out of the patients with UA, 17 were pre-menopausal, 21 were peri-menopausal, 84 were post-menopausal.
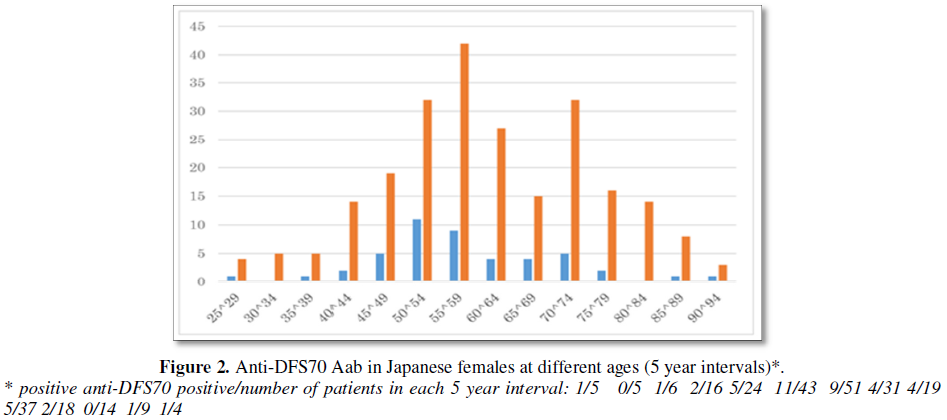
A positive ANA was found in 84.1% (237/282) patients. Anti-DFS70 Aab was found in 17.4% (49/282) of women (Figure 1a) and was detected in all age groups (Figure 2). When females younger than 30 and older than 80 years were excluded because of small numbers, more than 50% of positive anti-DFS70 Aab subjects were in the in 46 to 59 year old cohort. Frequencies of anti-DFS70 Aab in 45-49, 50-54 and 55-59 age groups were 18.5% (5/27), 25.5% (12/47) and 14.2% (7/49), respectively (Chi square test p=0.52, not statistically significant).
Frequency and anti-DFS70 Aab titers in pre-menopausal, peri-menopausal and post-menopausal women with UA
The frequency of anti-DFS70 Aab in 122 UA women was 17.6% (3/17) with PrMS, in 28.5% (6/21) with PeM and in 26.1% (22/84) with PoM (Table 1). The mean anti-DFS70 ELISA titers (absorbance index) of 8, 16.9 and 15.7 were observed in PrMS, PeMS and PoMS, respectively.
The frequency of anti-DFS70 Aab of 26.7% (28/105) in all PeM and PoM UA females was significantly higher than that of 10.8% in women with RA or 9.7% in women with primary SjS (p=0.013 by U test). Further, the mean ELISA index of 16.1 in PeMA and PoMA females was significantly higher than 8.1 in RA and 4.9 in primary SjS, respectively (Table 1). By comparison, 2 of 15 (13.3%) HI had anti-DFS 70 Aab and the observed frequencies of anti-DFS70 of 28.5% in UA PeMA and 26.1% in UA PoMA were higher than HI (not statistically significant) than in the other menstrual stages (PrMSA) studied here.
Anti-DFS70 Aab in pre-, peri- vs. post-menopausal women progressing to SARDs vs. non-SARD
The frequency of anti-DFS70 Aab of 41.2% (7/17) in PoMA associated with SARD was significantly higher than that the 14.7% (15/67) in PoMA without SARD (p<0.05) (Table 2). In addition, although the number of subjects is small, the frequency of anti-DFS70 Aab of 66.7% (2/3) in PeMA associated with ongoing to SARD is higher than that with 26.6% (4/18) in PeMA without SARD. The frequency of anti-DFS70 Aab of 37.5% (3/8) in PrMA with SARD is higher than that of 0% in 9 women without SARD (Correlation coefficient: y=0.1326x + 190.53; r=0.009).
Relationship between the ANA IIF titers of and anti-DFS70 ELISA among all 282 enrolled Japanese women
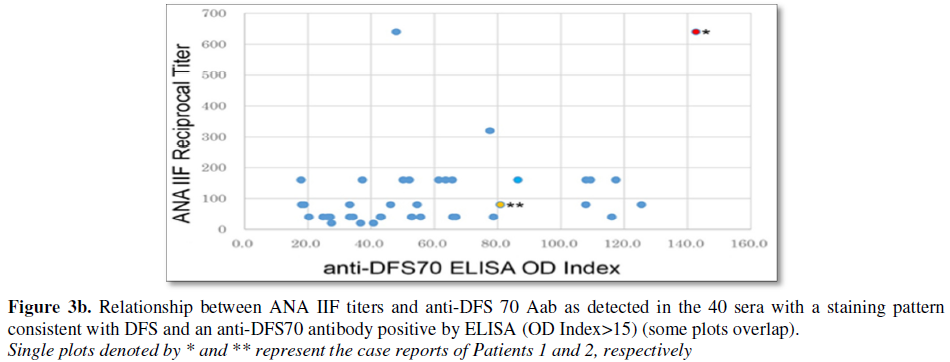
Anti-DFS70 Aab were found in 49/282 (17.3%) of all patients and in 49/237 (20.7%) of ANA positive patients (Figure 3a). There was a tendency for high titer ANA to be associated with low titer anti-DFS70 Aab by ELISA, and ANA low titer with high anti-DFS70 by ELISA (Figures 3a and 3b). Statistical analysis between ANA IIF titres and DFS70 antibodies revealed a correlation coefficient of r=0.357. In assessing ANA-related diseases, higher ANA IIF titers (³ 1:320) were associated with lower anti-DFS70 Aab titers in 3 of 10 (30%) SLE and 15 of 31 (48.4%) SjS patients.
The majority of anti-DFS70 Aab positive sera had a positive ANA at dilutions of 1:40-1:160. For example, 40/49 (81.6%) ANA positive with anti-DFS70 Aab sera showed anti-DFS (homogeneous/speckled) IIF staining patterns at the IIF screening dilution of ³ 1:40 (Figure 3b). The remainder of the IIF patterns included nuclear envelope (n=1), speckled (n=2), discrete speckled (n=2), PCNA-like (n=1) and negative (n=3).
DISCUSSION
We began with a presentation of two postmenopausal patients presenting with arthralgias in the context of a positive ANA by IIF and monospecific anti-DFS70 antibody. Despite a positive ANA, the rheumatologists did not find evidence for a SARD and their arthralgia was thought to be secondary to their post-menopausal state. Their symptoms were treated successfully with cyclic HRT for 2 months. Menopausal arthralgias not well understood, however, they are very common complaints in Japan, yet only 2~3% of these patients received HRT compared to 20 to 50% of menopausal women receiving HRT in USA, Europe and Australia [18]. HRT treatment in some jurisdictions in Japan is administered by general practitioners, gynecologists and internists, but uncommonly rheumatologists.
Our patients with menopausal joint pains achieved less than 5 points in the ACR/EULAR classification criteria for early RA and hence were classified as undifferentiated arthritis (UA) [11]. As previously reported in a Dutch study, over a one year follow-up, 40% of UA developed RA and 20% UA progressed to defined diseases such as Sjögren’s syndrome and 40% UA spontaneously resolved [14]. One of the prevailing concerns of rheumatologists is the use of an ANA in patients with ‘aches and pains’ (i.e., as in our patient 1) to rule out a diagnosis of SARD [19], However, it is precisely in this setting that the ANA can be helpful because if it is found to be monospecific anti-DFS70 it effectively rules out the diagnosis of a SARD [20,21] and can help allay patient anxiety and physician uncertainty [4,5]. Since HRT is not so effective in treating overt RA [22], it may be helpful in discriminating estrogen deficiency or depletion arthralgia (menopausal arthralgia) from early stage of RA. For example, in our experience HRT treatment of RA results in less than 30% reduction of the visual analog joint pain scale (unpublished).
In general, reports to date indicate that the frequency of anti-DFS70 is higher in younger females [23] and in apparently healthy individuals and individuals with SARD than it is in individuals with a SARD [4,8,24]. In our study, anti-DFS70 Aab was found in 16.3% of apparently healthy Japanese women, which is comparable to the frequency reported by Watanabe et al. [9], who, using an immunoblotting and ELISA, reported a frequency of 10.7% in a study of healthy Japanese hospital workers, 75.9% of which were females with a mean age of 31 years. In our study there was no significant difference in the frequency of anti-DFS70 Ab in females with UA at different stages of menopause as compared to HI and the frequency of anti-DFS70 Aab in females with UA associated with menopausal complaints (26.7%) was significantly higher than those with possible/probable or defined SARD (9.7% primary SjS; 10.8% RA). These observations are inconsistent with the study of Watanabe who reported anti-DFS Aab in only 1.5% of individuals with a SARD [25]. Since anti-DFS70 is found in healthy individuals as well as SARD, further study has shown that a key differentiating feature is that in healthy individuals the anti-DSF70 response tends to be restricted to that antigen whereas in SARD, the Aab are found in association with other SARD-related Aab [26]. In our two patients reported here no other SARD-related Aab were found.
The hypothesis that the estrogen hormonal status may be an explanation for anti-DFS70 B-cell responses was not proven by our study where the focus was on pre-, peri- or post-menopausal women who lacked a SARD. The anti-DFS70 frequency of ~27% in PeMA and PoMA patients in our study is among the highest published in adult or pediatric cohorts to date. Previous studies reported that the frequency of anti-DFS70 may be higher in younger females [9,23], especially in pre-pubertal children [10]. Although there was a trend to a lower frequency of anti-DFS in PrMA, our PrMA numbers were small because of the focus on menopausal women rather than PrMA and the youngest PrMA patient was 26 years of age.
A limitation of our study is that we did not determine if there was a correlation between anti-DFS70 Aab levels and estrogen/FSH levels. In addition, since our study did not focus on young or pre-menopausal Japanese females without arthralgia or SARD, further study on this population is required. In addition, earlier studies have reported anti-DFS Aab in in 3~30% of atopic dermatitis or alopecia [27] and in 10% of interstitial cystitis [6]. Moreover, it was found in ~30% of children with a positive ANA and chronic fatigue syndrome [28] and in patients with cataracts [7]. However, in our study, we did not systematically evaluate patients for these conditions. Although it has been reported that ani-DFS70 responses remain relatively stable over time [29,30], formal, well designed longitudinal studies are still required.
In our two case reports, we reported the ANA IIF description that was provided in the laboratory diagnostic report (homogenous and speckled). The ICAP nomenclature for DFS immunofluorescence pattern on HEp-2 cells (AC-2) has only recently been described on the website (https://anapatterns.org/index.php). Nevertheless, many laboratories do not report the DFS IIF pattern as described and one recent study showed that there continues to be significant inter-laboratory disparity in recognizing and reporting the DFS IIF pattern [31]. Indeed, if one compares the ICAP AC-1 and AC-2 patterns and descriptors they are very similar in that both stain the interphase nuclei and the metaphase chromatin. We confirmed the DFS (AC-2) pattern for both Patient 1 and Patient 2.
In our study, anti-DFS70 Aab was most commonly found in sera with ANA IIF titers of less than 1:160. It is well-known that the titers of anti-DFS70 as detected by IIF can be very high (i.e., >1/2560) [32]. ANA titers of 1:320 or greater were also associated with anti-DFS70 Aab in our study. However, only 6 of all 46 anti-DFS70 positive sera were positive for anti-DFS70 Aab (ELISA index >15) at an ANA of 1:320 or greater. These data suggest that anti-DFS70 Aab as detected by our ELISA that utilized a human recombinant protein is not highly correlated with conventional ANA IIF patterns, a finding that is consistent with previous reports showing that not all patients with anti-DFS70 have the complimentary DFS IIF staining pattern on HEp-2 cells [26,33].
Since anti-DFS70 Aab may be associated with the HLADRB1genetic background [34] that along within infections, stressor proteins and estradiol fluctuation or depletion might further enhance the autoimmune response [35]. Therefore, in addition to defining HLADRB1 status, future studies might survey patients for infections (i.e., microbiome) or stressor proteins [36] as well as hormonal status as possible contributors to the anti-DFS70 response. In addition, considering the possible role of hormones like estrogen on autoimmune responses, follow-up anti-DFS70 and ANA testing after HRT would be a potentially informative study. In this context, it is possible that menopausal related hormonal changes may be associated with viral reactivation and transient arthralgia and an anti-DFS70 response. Ongoing studies are hoping to determine if a viral (or bacterial) exposure triggers and perpetuates the anti-DFS70 response. Based on demographic data alluded to above; potential triggers might include Epstein Bar virus or the human papilloma virus. However, the human immunodeficiency virus may be more relevant because the N-terminus of DFS70 plays a role in the recruitment of the cognate DFS70 protein to chromatin and the C-terminus contains a highly conserved domain termed the human immunodeficiency virus integrase (HIV-IN) binding domain (IBD) that promotes the integration of HIV DNA into transcriptionally active chromatin [37]. Of note, the IBD also represents the predominant DFS70 epitope that binds human anti-DFS70 antibodies [37].
In summary, an important contribution of our study and supporting case reports is that in the management of menopausal females where joint pains can be a significant clinical component, an assessment of anti-DFS70 is an important consideration. In these patients, the finding of anti-DFS70 is an important step to ruling out a SARD and these patients can benefit from HRT. Further, our studies also support the evidence that the reliable detection of anti-DFS70 requires testing for anti-DFS70 Aab using ELISA or other analyte-specific assays [4,38]. If positive, unless the clinical picture changes, they should not continue visiting rheumatology clinics for evaluation SARD as part of a cost-effective approach to diagnostics [35].
ACKNOWLEDGEMENT
We thank for Dr. Takahashi for providing part of the anti-DFS70 ELISA kit (MBL).
CONFLICTS OF INTEREST
MJF received speaking and consulting honoraria from Inova Diagnostics (San Diego, CA) and research gifts in kind from Euroimmun GmbH (Luebeck, Germany).
The other authors have no conflict of interest.
1. Nagamine R, Maeda T, Shuto T, Nakashima Y, Hirata G, et al. (2001) Menopausal syndrome in female patients with rheumatoid arthritis. Mod Rheumatol 11: 230-233.
2. Chung E, Mo H, Wang S, Zu Y, Elfakhani M, et al. (2018) Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr Res 49: 23-36.
3. Miyachi K, Hankins RW, Uehara Y, Zhang B, Saku K, et al. (2009) A post-menopausal patient with Tangier disease developing Sjogren's syndrome. J Rheumatol 36: 208-210.
4. Conrad K, Rober N, Andrade LE, Mahler M (2017) The clinical relevance of anti-DFS70 autoantibodies. Clin Rev Allerg Immunol 52: 202-216.
5. Mahler M, Meroni PL, Andrade LE, Khamashta M, Bizzaro N, et al. (2016) Towards a better understanding of the clinical association of anti-DFS70 autoantibodies. Autoimmun Rev 15: 198-201.
6. Ochs RL, Muro Y, Si YZ, Ge H, Chan EKL, et al. (2000) Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allerg Clin Immunol 105: 1211-1220.
7. Singh DP, Ohguro N, Chylack LT Jr, Shinohara T, Lens, et al. (1999) Epithelium-derived growth factor: Increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci 40: 1444-1451.
8. Ochs RL, Mahler M, Basu A, Rios-Colon L, Sanchez TW, et al. (2016) The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: Integrating basic science with clinical understanding. Clin Exp Med 16: 273-293.
9. Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EA, et al. (2004) Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum 50: 892-900.
10. Schmeling H, Mahler M, Levy DM, Moore K, Stevens AM, et al. (2015) Autoantibodies to dense fine speckles in pediatric diseases and controls. J Rheumatol 42: 2419-2426.
11. van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH (2011) Classification of rheumatoid arthritis: Comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum 63: 37-42.
12. Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K, et al. (2004) Revised Japanese criteria for Sjogren's syndrome (1999): Availability and validity. Mod Rheumatol 14: 425-434.
13. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, et al. (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271-1277.
14. van der Helm-van Mil AH, Le CS, van DH, Breedveld FC, Toes RE, et al. (2007) A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: How to guide individual treatment decisions. Arthritis Rheum 56: 433-440.
15. Miyachi K, Ihara A, Sasse B (2018) Does hormone replacment therapy prevent undifferentiated arhtrits progressing to rheumatoid arthrits. EULAR2018 Final Program, THU0696.
16. Lobo RA, Pickar JH, Stevenson JC, Mack WJ, Hodis HN, et al. (2016) Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 254: 282-290.
17. Hawker GA, Mian S, Kendzerska T, French M (2011) Measures of adult pain: Visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken) 63: S240-S252.
18. Zbuk K, Anand SS (2012) Declining incidence of breast cancer after decreased use of hormone-replacement therapy: Magnitude and time lags in different countries. J Epidemiol Community Health 66: 1-7.
19. Abeles AM, Abeles M (2013) The clinical utility of a positive antinuclear antibody test result. Am J Med 126: 342-348.
20. Fitch-Rogalsky C, Steber W, Mahler M, Lupton T, Martin L, et al. (2014) Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. PLoS One 9: e93812.
21. Bonroy C, Schouwers S, Berth M, Stubbe M, Piette Y, et al.(2018) The importance of detecting anti-DFS70 in routine clinical practice: Comparison of different care settings. Clin Chem Lab Med 56: 1090-1099.
22. Holroyd CR, Edwards CJ (2009) The effects of hormone replacement therapy on autoimmune disease: Rheumatoid arthritis and systemic lupus erythematosus. Climacteric 12: 378-386.
23. Albesa R, Sachs UJ, Infantino M, Manfredi M, Benucci M, et al. (2018) Increased prevalence of anti-DFS70 antibodies in young females: Experience from large international multi-center study on blood donors. Clin Chem Lab Med.
24. Shovman O, Gilburd B, Chayat C, Amital H, Langevitz P, et al. (2018) Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin Exp Rheumatol 36: 121-126.
25. Kraemer DM, Kraus MR, Kneitz C, Tony HP (2003) Nucleoporin p62 antibodies in a case of mixed connective tissue disease. Clin Diagn Lab Immunol 10: 329-331.
26. Choi MY, Clarke AE, St PY, Hanly JG, Urowitz MB, et al. (2017) The prevalence and determinants of anti-DFS70 autoantibodies in an international inception cohort of systemic lupus erythematosus patients. Lupus 26: 1051-1059.
27. Okamoto M, Ogawa Y, Watanabe A, Sugiura K, Shimomura Y, et al. (2004) Autoantibodies to DFS70/LEDGF are increased in alopecia areata patients. J Autoimmun 23: 257-266.
28. Kuwabara N, Itoh Y, Igarshi T, Fukunaga Y (2009) Autoantibodies to lens epithelium-derived growth factor/transcription co-activator P75 (LEDGF/P75) in children with chronic nonspecific complaints and with positive antinuclear antibodies. Autoimmunity 42:492-496.
29. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, et al. (2011) Pattern on the anti-nuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum 63: 191-200.
30. Muro Y, Sugiura K, Nakashima R, Mimori T, Akiyama M, et al. (2013) Low prevalence of anti-DFS70/LEDGF antibodies in patients with dermatomyositis and other systemic autoimmune rheumatic diseases. J Rheumatol 40: 92-93.
31. Bentow C, Fritzler MJ, Mummert E, Mahler M (2016) Recognition of the dense fine speckled (DFS) pattern remains challenging: results from an international internet-based survey. Auto Immun Highlights 7: 8.
32. Dellavance A, Viana VST, Leon ER, Bonfa ESDO, Andrade LEC (2005) The clinical spectrum of antinuclear antibodies associated with the nuclear dense fine speckled immunofluorescence pattern. J Rheumatol 32: 2144-2149.
33. Miyara M, Albesa R, Charuel JL, El Amri M, Fritzler MJ, et al. (2013) Clinical phenotypes of patients with anti-DFS70/LEDGF antibodies in a routine ANA referral cohort. Clin Dev Immunol.
34. Muro Y, Ogawa Y, Sugiura K, Tomita Y (2006) HLA-associated production of anti-DFS70/LEDGF autoantibodies and systemic autoimmune disease. J Autoimmun 26: 252-257.
35. Murphy AJ, Guyre PM, Pioli PA (2010) Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol 184: 5029-5037.
36. Singh DP, Fatma N, Kimura A, Chylack LT Jr, Shinohara T, et al. (2001) LEDGF binds to heat shock and stress-related element to activate the expression of stress-related genes. Biochem Biophys Res Commun 283: 943-955.
37. Ogawa Y, Sugiura K, Watanabe A, Kunimatsu M, Mishima M, et al. (2004) Autoantigenicity of DFS70 is restricted to the conformational epitope of C-terminal alpha-helical domain. J Autoimmun 23: 221-231.
38. Seelig CA, Bauer O, Seelig HP (2016) Autoantibodies against DFS70/LEDGF exclusion markers for systemic autoimmune rheumatic diseases (SARD). Clin Lab 62: 499-517.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Allergy Research (ISSN:2642-326X)