895
Views & Citations10
Likes & Shares
In this study, Nostoc
carneum was taken as a model microorganism (cyanobacterium) for studying
the biodegradation efficiency of azo dyes. The degradation of methyl orange
(MO) and one of the commercial textile azo dyes (YA) were investigated.
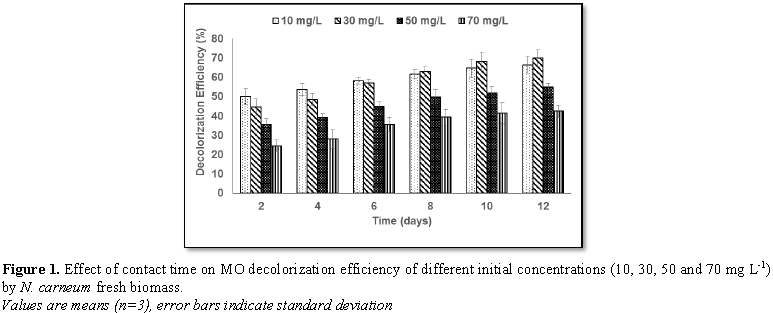
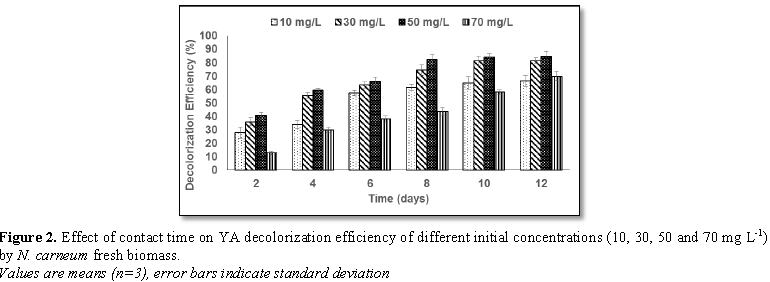
Decolorization efficiency (DE) increased progressively with contact time (12
days) for all concentrations of the two dyes (10, 30, 50 and 70 mg L-1).
The highest DE was observed with 30 mg L-1 for MO (69.8%) and 50 mg
L-1 YA (84.9%). There was a progressive increment in biomass with
both MO and YA dye supplementation. The maximum recorded biomass was 911.20 mg
L-1 for 30 mg L-1 MO and 746.57 mg L-1 for50
mg L-1 YA. The tow azo dyes enhanced chlorophyll a and carotenoids
production. 30 mg L-1 MO addition increased protein content while 50
mg L-1 YA supplementation promoted protein accumulation. N. carneum potentiality of producing
laccase enzyme for the two azo dyes degradation was investigated. There was
exponential relationship between dye concentration and laccase activity until
reached maximum laccase activity (14.848 U ml-1) for 70 mg L-1
MO and (22.592 U ml-1) for 70 mg L-1. According to the
UV-Vis. spectral analysis profile of culture filtrates, the mechanism by which N. carneum could decolorize the two
investigated azo dyes may be due to biosorption of dye molecules onto cell
surface as well as biodegradation by laccase. It is concluded that N. carneum could be an efficient
cyanobacterium for decolorization of dye effluents via adsorption and/or
biodegradation.
Keywords: Nostoc carneum, Azo dyes, Methyl orange,
Decolorization, Biodegradation, Laccase
INTRODUCTION
Nowadays the
aquatic ecosystem worldwide is subjected to many environmental challenges as a
result of the anthropogenic activities. Jin et al. [1] reported that wastewater
of the textile industry represented one of the principle sources of serious
pollution issues all over the world, since the textile industrial effluents
received many tons of textile dyes annually which causes severe risks according
to the dyes and their metabolites [2]. Azo dyes as xenobiotic pollutants are
members of the aromatic heterocyclic compounds possessing azo bond (–N=N–)
which compose most of the textile dyestuff discharged in many industries
demonstrating their impact in stability, toxicity and carcinogenicity [3].
Consequently, different physico-chemical methods of dyes remediation from
polluted wastewater were recommended such as coagulation, adsorption,
photo-degradation, ozonation and flocculation which varied in decolonization
efficiency as well as mechanistic capability of treatment. Though, the most
important criticism of these remediation protocols is that they only transfer
dyes between the partitioning phases of the remediation process without any
degradation [4]. In addition, these methods are highly cost, effective in the
low effluent volume and produced carcinogenic byproducts as documented by [5].
Bioremediation, the ecofriendly alternative process, is a promising method of
detoxification and recycling of industrial wastewater [6]. Dyedecolonization
potentiality was indicated for different microorganisms such as fungi,
bacteria, algae as well as their metabolic machinery for biodegradation [7]. As
reported by Jinqi and Houtian [8], biodegradation of azo dyes by microalgae are
influenced by the availability of dyes to be degraded depending on dye chemical
structure as well as the algal capability of dye removal in response to the
physiological characteristics of the microalga. Moreover, the
Enzymes having
protein containing cupper atoms are co-factors for some metabolic pathways of
fungi which incorporated in the catabolic pathways of some xenobiotic
pollutants. As indicated by Kiiskinen et al. [15], laccase enzyme is a member
of this cupper containing enzymes (polyphenol oxidases) represented the
oxidoreductases. Where the oxidation effect of laccase resulted in oxidation of
the substrate by losing an electron and the formation of a free radical mostly
takes place without production of toxic aromatic amines where cross linking or
depolymerisation reaction occurred. Some substrates may function as redox
mediators, permitting an indirect oxidation of some substances like
poly-aromatic hydrocarbons [16]. Currently, applications of laccase may be seen
as the start of an environmental versatile green catalyst in bioremediation
processes [4]. In the enzymatic pathway of synthetic dyes remediation, laccases
are the most effective enzymes where they are commonly extracellular and
catalyzes the oxidation of numerous phenolic compounds, thiols, aromatic amines
through an electron acceptor (molecular oxygen)as reported by Faraco et al.
[17]. These enzymes are preferred in biotechnological applications in numerous
industries and bioremediation of industrial pollutants due to their low
substrate specificity and their potentiality to act on chromophore group
compounds.
The current study
focused on studying the potential of Nostoc
carneum to decolorize and/or degrade methyl orange (MO) as a model azo dye
for investigating the mode of action of decolorization process and the enzymes
that may be involved for MO metabolism. Therefore, the main objectives of this
study are; to evaluate the potentiality of N.
carneum to decolorize MO; to follow growth of N. carneum in presence of MO and to monitor dye degradation via
assessing laccase activity.
MATERIALS AND METHODS
Biosorbent
Fresh biomass of
the Cyanobacterium, Nostoc carneum, that
grown in BG11 medium, was used as a biosorbent for studying methyl orange decolorization.
Algal cells were harvested at the beginning of stationary phase (18th
day).
Adsorbate
Methyl orange,
4-[4-(dimethyl amino) phenyl azo] benzene sulfonic acid, is a sulfonated
mono-azo dye having molecular formula C14H14N3NaO3S
was selected as an adsorbate (model dye). The yellow azo (YA) were one of the
commercial textile dyes used in laundries. The dye stock solution was adjusted
to the concentration 100 mg/L.
Dye biodegradation study
Batch scale
decolorization experiments were performed using axenic cultures of the
cyanobacteria N. carneum in glass
bottles of 0.5 L capacity as experimental cultivation units. In preparation for
the inoculation, N. carneum was grown
previously in BG11 medium (pH 7) for 14 days under static-incubation condition
and continuous illumination (3000 Lux) at 28 ± 2°C. The total volume of BG11
medium used was 240 ml and was inoculated with 10 ml of N. carneum culture (in the early stationary growth phase) and the
following concentrations of MO and YA dye were supplemented (10, 30, 50 and 70
mg L-1). Control treatment contained only growth medium and dye.
This control was used to ensure the photo-stability of dye solutions and/or no
adsorption occurred in the cultures vessels. Another control was used contained
only growth medium and inoculum to identify the changes in growth behavior
throughout the experiment [18]. A known volume of algal culture was filtered
using Millipore filter paper; then dried in an oven at about 70°C till a
constant weight was obtained.
Aliquots of cell
free culture filtrate were taken at time intervals of 2 days under sterile
conditions for decolorization assay. The samples were centrifuged at 4000 rpm
for 10 min residual dye concentration was calculated following a calibration
curve using Unico UV-2000 Spectrophotometer. The absorbance was measured at the
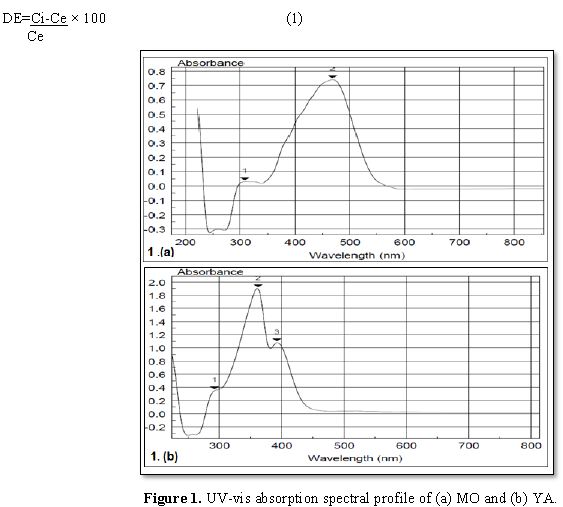
maximum wavelength of both MO dye (λmax=470 nm) and YA dye (λmax=362
nm) using scan spectrophotometer Unican UV/Vis (Figure 1). The samples
were discarded with time course intervals every two days up to 12 days. Dye
biodegradation was evaluated spectrophotometry (Unican UV/Vis, England) and the
decolorization efficiency was calculated as previously explained in Eq. 1.
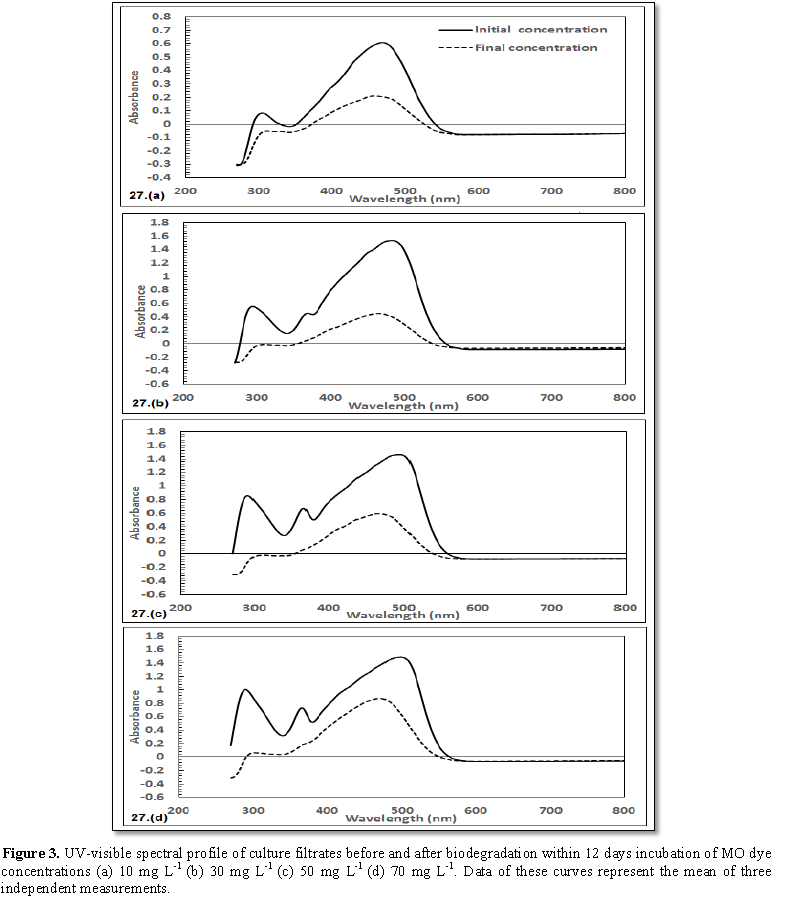
UV-scan profile was performed for culture filtrates before and after biodegradation
within 12 days incubation.
Determination of
photosynthetic pigments
A known volume of N. carneum culture was centrifuged at 4000 rpm for 10 min; algal
pellet was extracted with acetone. Chlorophyll a and carotenoids were estimated
according to Metzner et al. [19], using UNICO UV-2000 spectrophotometer at
wavelengths 470, 645 and 662 nm. Pigments were calculated according to the
following equations:
Chlorophyll a=11.75 λ662-2.350 λ645
Chlorophyll b=18.6 λ645-3.960 λ662
Carotenoids=(1000 λ470-2.270 chl.a
– 81.4 chl.b)/227
A known volume of N. carneum culture was centrifuged at 4000 rpm for 10 min, 5 ml
buffer pH=6.7 were added to the algal pellet, extraction was performed using
ultrasonication (Sinacator: Col-Pormer instrument Co Chicago, Illinois 60648,
USA), finally the extract was centrifuged to get rid of algal depresses.
Phycobilli proteins were calculated according to the following equations:
Phycocyanin (PC)=λ615 – (0.474 × λ652/5.34)
Allophytocyanin (APC)=λ652 –
(0.208 × λ615/5.09)
Phycoerithrin (PE)=λ562 – (0.241 × PC) –
(0.849 × APC) ÷ 9.62
Determination of
protein content
Protein content was estimated according to
Lowry et al. [20] using crystalline bovine serum albumin as standard. Culture
aliquot of 50 ml was centrifuged and the biomass pellet was extracted, using
phosphate buffer (pH 7). Protein is estimated using phenol Folin reagent.
Determination of
laccase activity
Fresh N.
carneum biomass was collected by centrifugation of certain aliquot of algal
culture at 4000 rpm for 15 min. Laccase crude preparation was extracted using
0.2 mM sodium phosphate buffer pH 7.0. To estimate laccase activity, guaiacol
used as a substrate, the following assay procedures were achieved. 2 ml of 100
mM acetate buffer (pH 4.5), 1 ml, 50 mM sodium malonate, in addition to 1 ml of
10 mM guaiacol and the reaction was initiated by supplying 1 ml enzyme
preparation then incubated at room temperature for 10 min to ensure the
developing of brown color. The brown color development was attributed to the
oxidative polymerization of substrate (guaiacol) which verifying the presence
of laccase. The phenolic product formation was determined by measuring the
absorbance at 530 nm with extension coefficient (ε=6740 M-1cm-1)
as documented by Jhadav et al. [21]. The absorbance for the negative control
was estimated at 470 nm. The enzyme activity was calculated as U ml-1
which represented the amount of enzyme needed to produce 1 μM product min-1
at 30°C.
RESULTS
UV-vis spectral
analysis of MO and YA decolorization by N.
carneum
The present data indicated that N. carneum had the ability to decolorize
the two azo dyes in BG11 medium. Data (Figures
2 and 3) indicated that the decolorization efficiency (DE) increased
progressively with contact time for all concentrations of the two dyes. The
highest DE was noticed with 30 mg L-1 MO (69.8%) and 50 mg L-1
YA (84.9%).The decolorization process was verified by UV-scan spectroscopy
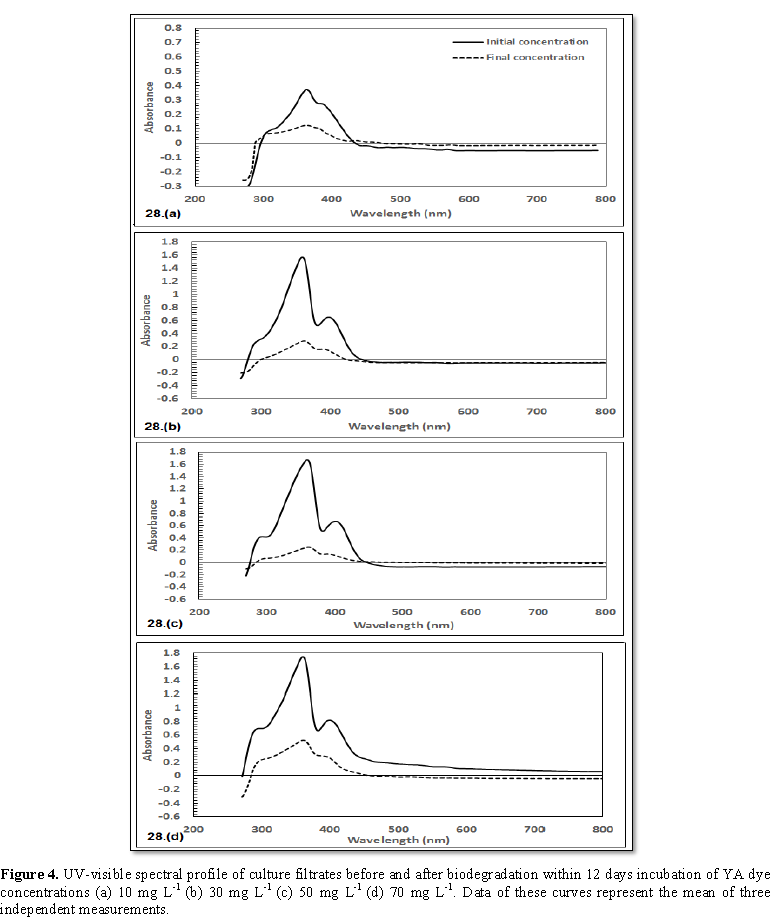
presented in Figures 4 and 5 for MO
and Figure 5 for YA. Generally, λmax of all concentrations exhibit
small move in the direction of lower wavelength (Table 1). In case of MO (10 mg L-1), the spectral beak
at 306 nm (A=0.083 nm) disappeared. For 30 mg L-1 MO, the two beaks
that could be concerned at λ294 nm (A=0.556 nm) and λ368
nm (A=0.455 nm), disappeared. With respect to 50 mg L-1 MO
supplementation, two spectral beaks were recognized at λ292 nm
(A=0.0854 nm) and at λ368 nm (A=0.065 nm) disappeared. In case of 70
mg L-1 MO addition, there were two beaks detected at λ292
nm (A=0.099 nm) was shifted to λ298 nm (A=0.062 nm) and the other
was detected at λ366 nm (A=0.073 nm) appeared with reduced
absorbance (A=0.018 nm) concerning YA, a comparable behavior was attained where
beaks (λ312 nm with A=0.095 nm as well as λ390 nm with
A=0.261 nm) were disappeared in case of 10 mg L-1. The same pattern
of response, in case of 30 mg L-1 YA addition, the two beaks λ294
nm (A=0.286 nm) and λ400 nm (A=0.645 nm) were dissipated addition.
The same trend was repeated in case of 50 mg L-1 YA supplementation
where beaks at λ290 nm (A=0.412 nm) as well as λ406 nm
(A=0.650 nm) could not be detected. Finally, 70 mg L-1 YA addition,
spectral beak at λ292 nm (A=0.693 nm) exhibited decreased absorption
(A=0.224 nm) with shifting in wavelength to λ296 nm. Photos 1a and 1b indicated deep colored
biomass in cultures of either MO or YA in all performed concentrations.
Physiological study
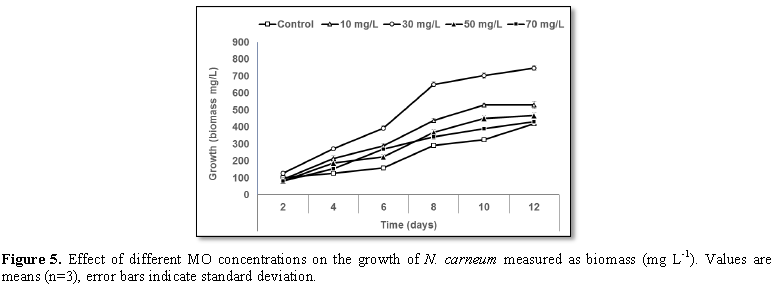
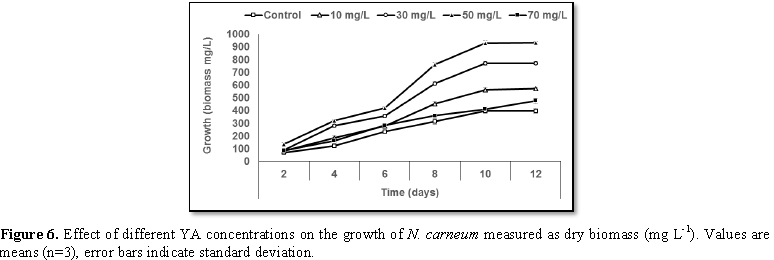
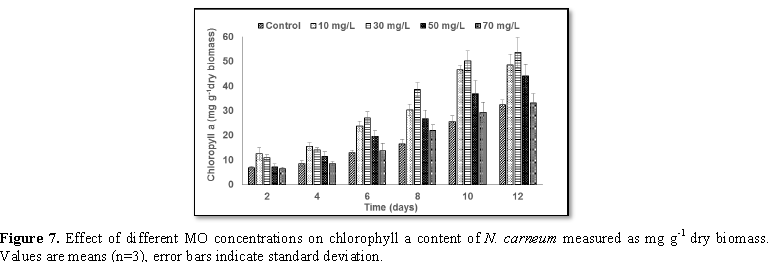
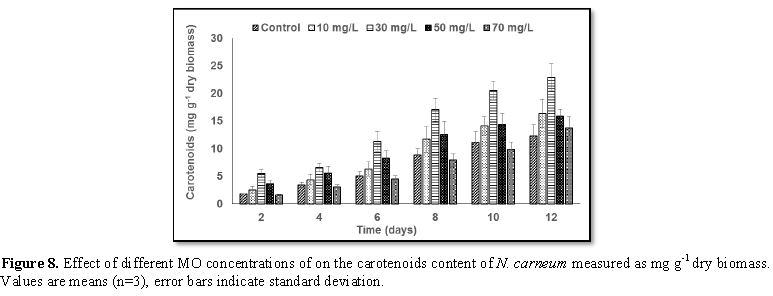
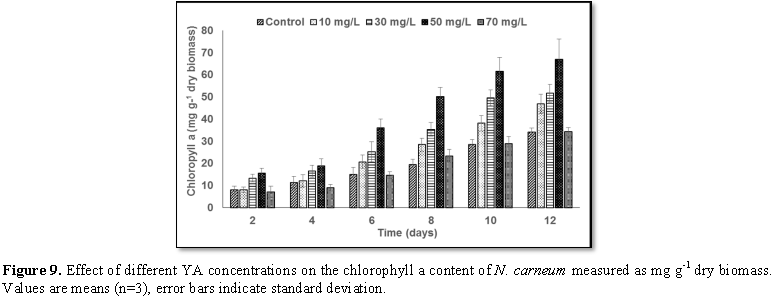
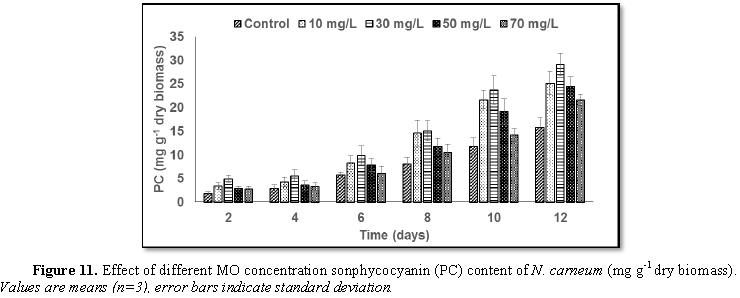
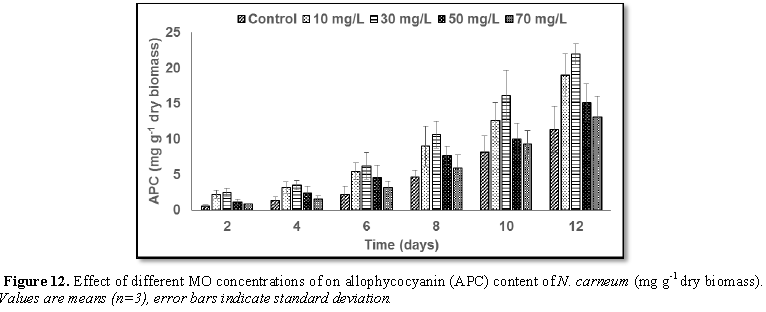
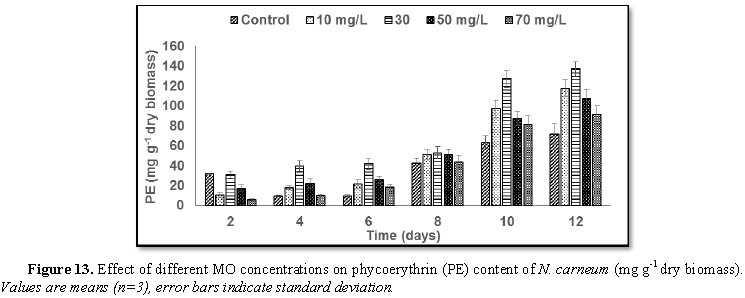
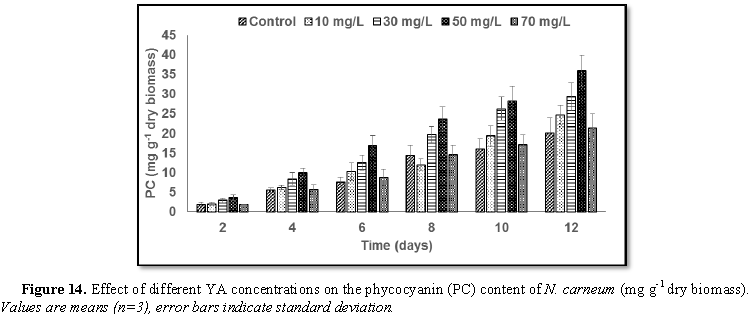
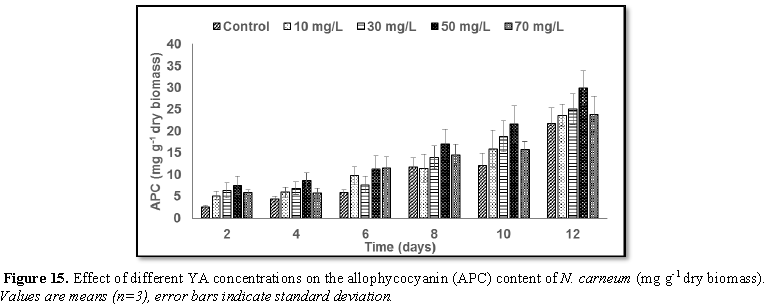
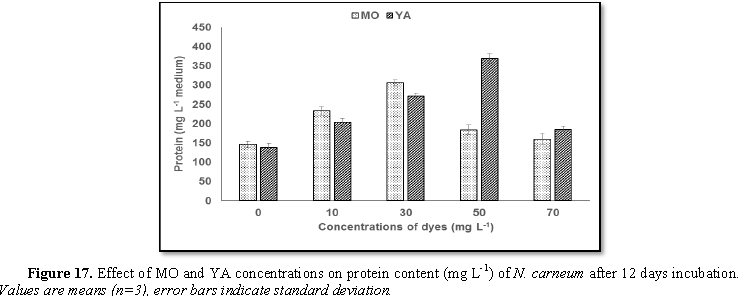
Stimulatory growth pattern was illustrated in Figures 6 and 7 showing a progressive increments in biomass with both MO and YA dye supplementation throughout the experimental period. With respect to MO supplementation the following descending order was attained 30>10>50>70 mg L-1, while the following descending order was allowed to YA addition 50>30>10>70 mg L-1. The maximum biomass was recorded to 30 mg L-1 for MO (911.20 mg L-1 dry tissue) and 50 mg L-1 YA (746.57 mg L-1 dry tissue).Chlorophyll a and carotenoids are significant parameters for growth assessment which increased progressively throughout time course of experiment. The present results showed significant increments in these parameters with all dye supplementations as well as total photosynthetic pigments which gradually increased throughout the incubation period. MO induced maximum chlorophyll a content at the 10th day (0.0328 mg g-1 dry biomass) for 30 mg L-1 addition, while carotenoids content reached its maximum value at 12th day (6.6972 mg g-1 dry biomass) for 70 mg L-1 as indicated in Figures 8 and 9. Data presented in Figures 10 and 11 indicated that chlorophyll a and carotenoids contents as affected by YA supplementations, exhibited the same pattern of response with maximum chlorophyll a content at the 10th day (0.0382 mg g-1 dry biomass) for 50 mg L-1 addition and carotenoids content reached its maximum value at 12th day (6.7142 mg g-1 dry biomass) for 70 mg L-1 supplementation.In the current study, 30 mg L-1 MO addition induced the highest content of PC, APC and PE during the experimental period where the magnitude of response was proportional to MO concentrations giving the highest value at the end of the incubation period (29.09, 21.95 and 137.01 mg g-1 dry biomass, respectively) as illustrated in Figures 12-17. The same pattern of response was recorded, with respect to the effect of YA on PC, APC and PE contents, 35.91, 29.83 and 140.50 mg g-1 dry biomass as the maximum values of phycobilli proteins, respectively (Figures 14 and 16). In general, PE occupied the first level within the phycobilli protein pigments in all treated cultures with either MO or YA.
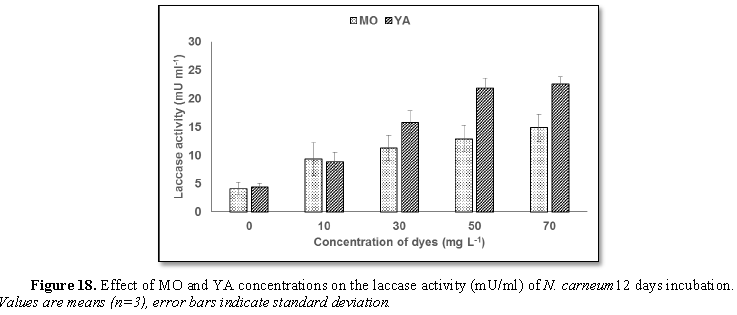
The obtained data (Figure 19) demonstrated that laccase activity exhibited higher
activity in case of YA supplementation than MO at the beginning of stationary
phase (12th day). It was shown that laccase activity was in
accordance with N. carneum growth
(biomass production) which interpreted the decolorization pattern of MO and YA
(Figures 3 and 4, respectively) on
basis of dyes oxidation which illustrated an exponential relationship between
dye concentration and laccase activity until reached maximum laccase activity
(14.85 U ml-1) for 70 mg L-1 MO and (22.59 U ml-1)
for 70 mg L-1 YA
DISCUSSION
Bioremediation activity of algae varied with
different dyes and this may be interpreted according to adsorption or/and
biodegradation by algae. Therefore, Acuner and Dilek [22] recommended that dye
characters such as chemical formula, electric charge as well as adaptation
period were significant factors affecting dye bioremediation. The current study
exhibit cyanobacteria-induced decoloration and degradation of MO and YA dyes by
N. carneum. Some azo dyes (methyl
red, orange II and G-Red) represented carbon source for some algae as reported
by Kulla et al. [23]. Algae could reduce azo dyes by cleavage the azo bridges
by the effect of azo reductase where aromatic amine formed as resulted
metabolite. Bacteria had a similar mechanism in degrading azo dyes resulted in
formation of aromatic amine [24]. According to Legerska et al. [25] suggestion,
the metabolites formation and the shifting of recorded λmax of
UV-visible scan could be pointed to dye degradation. Decreases in absorbance
might be due to the broken down of the dye chromophores (–NO2,
–N=N–, –NH2) and merged aromatic rings with formation of UV
absorbing intermediates as indicated by Parikh and Madamwar [10]. Likewise Kulla
et al. [23] reported that Pseudomonas strain could fully metabolize some
C14-labeled aromatic amines to carbon dioxide, in which many of these compounds
could be utilized by some algae as documented by Wang et al. [26]. According to
Shah et al. [27], decolorization of azo dye solution using Pseudomonas spp.
ETL-M might be attributed to either adsorption onto bacterial cell wall or to
biodegradation. In accordance of the present results, Pourbabaei et al. [28]
documented that MO was decolorized by Bacillus
cereus within 2 days of incubation in aerobic cultures. These findings are
in agreement with the current data which demonstrated that N. carneum could degrade 69.8% of MO and 84.9% of YA. There were
disappearance of some spectral beaks as well as darkening in biomass in case of
the two used dyes; this indicated that the resulted dye removal could be
attributed to biosorption activity and/or biodegradation. The present data
indicated the tolerance of N. carneum
to high concentration (70 mg L-1) of both dyes. The mechanism by
which N. carneum could decolorize the
two investigated azo dyes might be due to biosorption of dye molecules onto
cell surface and biodegradation as suggested by Ayed et al. [29]. Dellamatrice
et al. [30] studied the degradation of the following azo dyes; Indigo, Remazol
Brilliant Blue R and Sulphur Black using Anabeanaflos
aquae UTCC64, Phormidium autumnale UTEX1580
and Synechococcus sp. PCC7942 as
biosorbents. They found that the investigated cyanobacteria exhibited color
bioremoval potentiality and biodegradation activity as well as they concluded
that the degree of either decolorization or dye degradation was influenced by
both the cyanobacterial species used and dye structure. El-Sheekh et al. [31]
studied the capability of Pseudoanabaena
sp. and Microcystis aeruginosa for
biodegradation of the following dyes; Disp. orange (2RL), Reactive yellow
(3RN), Reactive Black (NN) and Tracid Red (BS) and found that the
biodegradation activity were reliant to the kind of dyes and the species of
algae used. Moreover, De Philippis et al. [32] documented that large number of
cyanobacteria had an outer membrane with supplementary outer substance such as
polysaccharide that were capable of pollutant elimination from aquatic system.
The ability of microorganisms to remove dyes might be in cause of adsorption
onto algal biomass or algal biodegradation [33]. Decolorization of azo dye
might be due to partial reduction or complete breaking down the azo bond as
noticed by Chang et al. [34].
As supplementing N. carneum culture with different concentrations of the
investigated azo dyes, the intracellular nutrients could be transported across
the cell membrane which forcing cells to metabolize the molecules of azo dyes
as external nutrient sources earlier in order to initiate both decolorization
as well as algal biomass production. Moreover, on the basis of the passive
diffusion and the influence of higher concentration gradient as a driving
forces from transporting azo dyes molecules from the exterior of bacterial
cells to their interior across plasma membrane. Mohanty et al. [35] interpreted
the enhancement of MO decolorization as well as Pseudomonase luteola growth in the MO supplemented cultures. Owing
to the spontaneous passive diffusion, relatively higher azo dye concentrations
were more preferred to penetrate to cell via the plasma membrane. El-Sheekh et
al. [24] who studied the effect of different dyes concentrations (methyl red,
orange II, G-Red (FN-3G), basic cationic and basic fuchsin) on growth of microalgae
(Chlorella vulgaris, Lyngbya lagerlerimi, Nostoc lincki, Oscillatoria rubescens, Elkatothrix
viridis and Volvox aureus). They
found significant decreases in dry weight production of the tested algae as
compared with control by increasing incubation time. Whereas, El-Sheekh et al.
[31] found that the growth of Microcystis
aeruginosa and Pseudoanabaena sp.
was decreased as a result of the effect of different dyes supplementations
compared to control where, there was little progressive increase in growth
during incubation period that was in accordance with dyes degradation. In the
same context, Yadav et al. [36] demonstrated that the indigo dye exhibited a
negative growth response as well as biomass production of Chlorella vulgaris at
different concentrations where, they deduced this pattern of response to the
ability of indigo to deal with microalgae by three strategies: additive,
synergistic or antagonistic to affect growth as well as chemical constituent of
biomass.
Mahalakshmi et al. [37] reported that either
Congo red or textile dye effluent increased chlorophyll a content in Chlorella
sp. with different dye concentrations (2, 5, 10, 12 and 15 ppm). On
investigating tolerance of Spirulina
spp., Lyngbya sp., Phormedium sp. and Synechocyctis sp. to methyl red (MR) supplementation (25-100 mg L-1)
for 18 days incubation period, Ansari et al. [4] found photosynthetic pigments
reduction in some cultures. Moreover, presence of MR induced some increments in
pigments, whereas Vajpayee et al. [38] explained the evolution of adaptive
mechanism for tolerance, where there was an enhancement of photosynthetic
pigment production in Spirulina – C11 cultures supplemented with MR recording
high degree of adaptation. On the other hand, Ali et al. [11] studied the decolorization
of Amido Black dye using marine Oscillatoria
formosa, chlorophyll a content decreased in all experimental treatments
compared to control which attributed to decline in the biosynthesis of
pigments. The same pattern of response was reported by Parikh and Madamwar [10]
where they found that Gloeocapsa
pleurocapsoides and Phormidium
ceylanicum could degrade about 80% of both Acid Red 97 and FF Sky Blue dyes
after 26 days incubation. This response could attribute to the harmful impact
of the dye addition of the photosynthetic apparatus (thylakoid bands) and the
biosynthesis of pigments as explained by Vajpayee et al. [38].
The obtained results for phycobilli proteins
were agreed with that of Mona and Kaushik [9] who studied the phycobilli
protein contents of the cyanobacterial species (Nostoc linckia HA-46, Myxosarcina
spectabilis HP-43 and Gloeocapsa
calcarea HP-45) as affected by different concentrations of reactive dye red
198. They documented that phycobilli protein pigments responded positively with
the increase of dye concentration as compared to control. The biomass of algal
periphyton decreased to about 40% of the initial mass after culture exposed to
high concentration of crystal violet (CV), where decolorization of CV was found
to be a synergistic process in combination with adsorption one ending with
biodegradation mechanism resulting in non-toxic aliphatic molecules as
documented by Shabbir et al. [39]. It is concluded that N. carneum might be try to safe its photosynthetic pigment system
from the adverse effects of the studied dyes as well as to evolve of defense
mechanism for tolerating the toxic effects of dyes as suggested by Stratton and
Corke [40] and Vajpayee et al. [38], where dyes addition improved biomass
production, chlorophyll a and phycobilli proteins as well. These results may
suggest the presence of some tolerance mechanism, since the high content of
phycobilli proteins in case of MO and YA N.
carneum supplemented cultures referred to an effective photosynthesis
process via effectual energy transference. Many studies demonstrated inhibitory
consequences in response to dye addition on microalgal thylakoid membranes and
biosynthesis of chlorophyll. Moreover, the current results indicated high level
of carotenoids and billi proteins with dye addition which may reflect the
capability of N. carneum to tolerate
presence of azo dyes [38].
According to Mahalakshmi et al. [37], the
protein content was not much affected by different concentrations of Congo red
where increased in Chlorella sp.,
Arthrospira as well as Hematococcus with dye supplementations even more than
control as detected in Chlorella sp.
with the same response pattern. Owing to Prabhakar and Krishna [41], the
aromatic amines were the principle metabolite resulted from the degradation of
azo dye. The observed dark colored biomass in the present study might be
attributed to the sensitivity of aromatic amines to auto oxidation resulted in
the production of dimers as suggested by Kudlich et al. [42]. Increasing
protein content in MR supplemented Spirulina-C11 could be referred to de novo
biosynthesis of some phenol-decomposing enzymes as well as presence of some
stress-associated proteins as a result of the presence of aromatic compounds
[43].
According to Telke et al. [44] and Legerská
et al. [45], azo dyes could be broken either symmetrically or asymmetrically in
the enzymatic degradation pathway by a highly non-specific free radical
mechanism producing phenolic compounds with hydroxy-, carboxy-, methoxy-,
amino- or sulpho- functional groups and might be applied as laccase activity
for bioremediation. Chivukula and Renganathan [46] previously proposed that
laccases could only transform a limited spectrum of azo dyes, specially dyes
that possess a phenolic substituent in para-position to the azo bond and
further methyl- or methoxy-substituents in 2- or 2,6-position relatively to the
hydroxy-group. In try to interpret the laccase function on azo dye degradation
and detoxification, Telke et al. [47] suggested that azo dye degradation
according to laccase action beginning with asymmetrically broken down of the
azo bond, then oxidative broken down, deamination, demethylation, desulfonation
and dihydroxylation depending on dye chemical formula. On the other hand, Chen
[48] explained azo dyes degradation without breaking down of the azo bond and
attributed the production of phenolic compound to the action of highly
non-specific free radical mechanism. An explanation of the mechanism by which
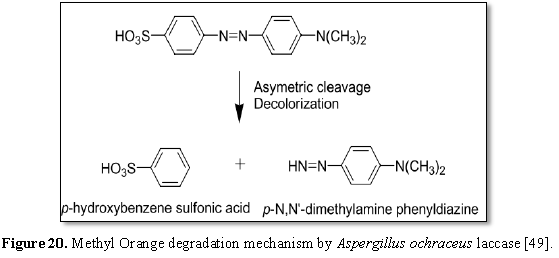
azo dye (MO) could be degraded by laccases produced by Aspergillus ochraceus NCIM-1146 was illustrated by Telke et al.
[49] in Figure 20. Firstly, laccases
degraded MO (mono azo dye) by carbocation, followed by production of an
electron-deficient reaction centre and consequently, extremely reactive
intermediates according to the presence of nucleophilic attack by -OH, -SO3
or halogen nucleophiles produced in asymmetric azo bond breakdown producing
p-N,N'-dimethylamine phenyldiazine and p-hydroxybenzene sulfonic acid. In spite
of toxicity of these products [50], the biological degradation of MO in aqueous
solution by Aeromonas sp. strain DH-6
possessing reduced phytotoxicty when as documented by Du et al. [51].
The potential of laccase induction by
different cyanoacterial species had been investigated by Ansari et al. [4]
using azo dye (MR). They found an enhanced induction in laccase activity
attributed to the addition of MR. Afreen and Fatma [52] reported that laccase
activity of Synechocystis NCCU-370 characterized by low activity in absence of
aromatic inducer giving its maximum activity (25.37 mU/ml) after 7 days of
incubation. Laccase activity was investigated in many fungi and bacteria where,
Patel et al. [53] found that 1 mM guaiacol addition improved laccase production
in many fungi as Phlebia spp. and Pleurotus ostreatus, whereas 8 mM
guaiacol had been suggested to be good laccase inducer in case of Rhodococcus sp. and Enterobacter sp. as demonstrated by Mongkolthanaruk et al. [54].
Laccase activity exhibited increment with increasing growth (biomass) that
recommended the necessity of appropriate growth for laccase induction, since
reduction in laccase activity and/or growth of Spirulina plantesis might be according to nutrients depletion as a
consequent result of aging where the maximum laccase activity was recorded on
the 10th day of incubation as indicated by Afreen et al. [55].
It is concluded that N. carneum exhibited decolorization activity via degradation
mechanism rather than adsorption one according to its adaptive nature as well
as degrading ability of azo dyes contaminants in addition to its tolerance and
decolorizing potentiality to relatively high concentration of MO which
recommended this cyanobacterium for bioremediation process of textile
wastewater.
1. Jin XC, Liu GQ, Xu ZH, Tao WY
(2007). Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. Appl Microbiol Biotechnol 74: 239-243.
2. Ozdemir S, Cirik K, Akman D,
Sahinkaya E, Cinar O (2013) Treatment of azo dye-containing synthetic textile
dye effluent using sulfidogenic anaerobic baffled reactor. Bioresour Technol
146: 135-143.
3. Sharma S, Sharma S, Singh P, Swami
R, Sharma K (2009) Exploring fish bioassay of textile dye wastewaters and their
selected constituents in terms of mortality and erythrocyte disorders. Bull
Environ Contam Toxicol 83: 29-34.
4. Ansari M, Khatib U, Owens G, Fatma
T (2016) Evaluation of Methyl Red tolerant cyanobacteria for simultaneous
laccase production and dye decolorization. Int J Waste Resour 6: 2.
5. Yang Y, Wang G, Wang B, Li Z, Jia
X (2011). Biosorption of Acid Black 172 and Congo Red from aqueous solution by
nonviable Penicillium YW 01: Kinetic study, equilibrium isotherm and artificial
neural network modeling. Bioresour Technol 102: 828-834.
6. Hu E, Shang S, Tao XM, Jiang S,
Chiu KL (2016) Regeneration and reuse of highly polluting textile dyeing
effluents through catalytic ozonation with carbon aerogel catalysts. J Clean
Prod 137: 1055-1065.
7. Olukanni O, Osuntoki A, Gbenle G
(2006) Textile effluent biodegradation potentials of textile effluent-adapted
and non-adapted bacteria. Afr J Biotechnol 5.
8. Jinqi L, Houtian L (1992)
Degradation of azo dyes by algae. Environ Pollut 75: 273-278.
9. Mona S, Kaushik A (2015) Screening
metal-dye-tolerant photoautotrophic microbes from textile wastewaters for
biohydrogen production. J Appl Phycol 27: 1185-1194.
10. Parikh A, Madamwar D (2005)
Textile dye decolorization using cyanobacteria. Biotechnol Lett 27: 323-326.
11. Ali DM, Suresh A, Kumar AP,
Gunasekaran M, Thajuddin N (2011) Efficiency of textile dye decolorization by
marine cyanobacterium, Oscillatoria
formosa NTDM02. Afr J Basic Appl Sci 3: 9-13.
12. Pourbabaei AA, Malekzadeh F,
Mohajeri A (2005) Decolorization of methyl orange (as a model azo dye) by the
newly discovered Bacillus sp. Iran J
Chem Chem Eng 5: 41-45.
13. Shah M, Patel K, Nair S, Darji A
(2013) Microbial decolourization of methyl orange dye by Pseudomonas spp. OA Biotechnol 2: 10.
14. Subasioglu T, Bilkay IS (2009)
Determination of biosorption conditions of Methyl Orange by Humicola fuscoatra. Journal of
Scientific and Industrial Research 68: 1075-1077.
15. Kiiskinen LL (2005)
Characterization and heterologous production of a novel laccase from Melanocarpus albomyces. VTT Technical
Research Centre of Finland.
16. Morozova O, Shumakovich G,
Gorbacheva M, Shleev S, Yaropolov A (2007) “Blue” laccases. Biochemistry
(Moscow) 72: 1136-1150.
17. Faraco V, Pezzella C, Miele A,
Giardina P, Sannia G (2009) Bio-remediation of colored industrial wastewaters
by the white-rot fungi Phanerochaete
chrysosporium and Pleurotus ostreatus
and their enzymes. Biodegradation 20: 209-220.
18. Hernández-Zamora M,
Cristiani-Urbina E, Martínez-Jerónimo F, Perales-Vela HV, Ponce-Noyola T (2015)
Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris. Environ Sci Pollut Res 22: 10811-10823.
19. Metzner H, Rau H, Senger H (1965)
Untersuchungen zur synchronisierbarkeit einzelner pigmentmangel-mutanten von
Chlorella. Planta 65: 186-194.
20. Lowry OH, Rosebrough NJ, Farr AL,
Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol
Chem 193: 265-275.
21. Jhadav A, Vamsi K, Khairnar Y,
Boraste A, Gupta N, et al. (2009). Optimization of production and partial
purification of laccase by Phanerochaete
chrysosporium using submerged fermentation. Int J Microbiol Res 1: 9.
22. Acuner E, Dilek F (2004) Treatment
of tectilon yellow 2G by Chlorella
vulgaris. Process Biochem 39: 623-631.
23. Kulla HG, Klausener F, Meyer U,
Lüdeke B, Leisinger T (1983). Interference of aromatic sulfo groups in the
microbial degradation of the azo dyes Orange I and Orange II. Arch Microbiol
135: 1-7.
24. El-Sheekh MM, Gharieb M,
Abou-El-Souod G (2009) Biodegradation of dyes by some green algae and
cyanobacteria. Int Biodeterioration Biodegradation 63: 699-704.
25. Legerská B, Chmelová D, Ondrejovič
M (2016) Degradation of synthetic dyes by laccases - A mini review. Nova
Biotechnologica et Chimica 15: 90-106.
26. Wang L, Zhang C, Wu F, Deng N
(2007) Photodegradation of aniline in aqueous suspensions of microalgae. J
Photochem Photobiol B Biol 87: 49-57.
27. Shah M, Patel K, Nair S, Darji A
(2013) Microbial decolorization of methyl orange dye by Pseudomonas spp. OA Biotechnol 2: 10.
28. Pourbabaei AA, Malekzadeh F,
Mohajeri A (2005) Decolorization of methyl orange (as a model azo dye) by the
newly discovered Bacillus sp. Iran J
Chem Chem Eng 5: 41-45.
29. Ayed L, Chaieb K, Cheref A,
Bakhrouf A (2009) Biodegradation of triphenylmethane dye Malachite Green by Sphingomonas paucimobilis. World J
Microbiol Biotechnol 25: 705.
30. Dellamatrice PM, Silva-Stenico ME,
de Moraes LAB, Fiore MF, Monteiro RTR (2017) Degradation of textile dyes by
cyanobacteria. Braz J Microbiol 48: 25-31.
31. El-Sheekh MM, Abou-El-Souod GW, El
Asrag H (2017) Biodegradation of some dyes by the micro algal species
(cyanobacteria) Pseudoanabaena sp.
and Microcystis aeruginosa. Egypt J
Exp Biol (Botany) 13: 233-243.
32. De Philippis R, Sili C, Paperi R,
Vincenzini M (2001). Exopolysaccharide-producing cyanobacteria and their
possible exploitation: A review. J Appl Phycol 13: 293-299.
33. Chen KC, Wu JY, Liou DJ, Hwang SCJ
(2003) Decolorization of the textile dyes by newly isolated bacterial strains.
J Biotechnol 101: 57-68.
34. Chang JS, Chou C, Lin YC, Lin PJ,
Ho JY, et al. (2001) Kinetic characteristics of bacterial azo-dye
decolorization by Pseudomonas luteola.
Water Res 35: 2841-2850.
35. Mohanty K, Jha M, Meikap B, Biswas
M (2006) Biosorption of Cr (VI) from aqueous solutions by Eichhornia crassipes. Chem Eng J 117: 71-77.
36. Yadav S, Srivastava V, Banerjee S,
Weng CH, Sharma YC (2013) Adsorption characteristics of modified sand for the
removal of hexavalent chromium ions from aqueous solutions: Kinetic,
thermodynamic and equilibrium studies. Catena 100: 120-127.
37. Mahalakshmi S, Lakshmi D, Menaga U
(2015) Biodegradation of different concentration of dye (Congo Red Dye) by
using green and blue green Algae. Int J Environ Res 9: 735-744.
38. Vajpayee P, Tripathi R, Rai U, Ali
M, Singh S (2000) Chromium (VI) accumulation reduces chlorophyll biosynthesis,
nitrate reductase activity and protein content in Nymphaea alba L. Chemosphere 41: 1075-1082.
39. Shabbir S, Faheem M, Wu Y (2018).
Decolorization of high concentration crystal violet by periphyton bioreactors
and potential of effluent reuse for agricultural purposes. J Clean Prod 170:
425-436.
40. Stratton GW, Corke CT (1979) The
effect of nickel on the growth, photosynthesis and nitrogenase activity of Anabaena inaequalis. Can J Microbiol 25:
1094-1099.
41. Prabhakar C, Krishna P (2013)
Bioremediation of textile dyes and improvement of plant growth by marine
bacteria. Int J Curr Microbiol Appl Sci 3: 962-970.
42. Kudlich M, Hetheridge MJ,
Knackmuss HJ, and A. Stolz (1999). Autoxidation reactions of different aromatic
o-aminohydroxynaphthalenes that are formed during the anaerobic reduction of
sulfonated azo dyes. Environ Sci Technol 33: 896-901.
43. Yemendzhiev H, Alexieva Z,
Krastanov A (2009) Decolorization of synthetic dye Reactive Blue 4 by mycelial
culture of white-rot fungi Trametes
versicolor 1. Biotechnol Biotechnol Equipment 23: 230-232.
44. Telke AA, Kalyani DC, Dawkar VV,
Govindwar SP (2009) Influence of organic and inorganic compounds on
oxidoreductive decolorization of sulfonated azo dye CI Reactive Orange 16. J
Hazard Mater 172: 298-309.
45. Legerská B, Chmelová D, Ondrejovič
M (2016) Degradation of synthetic dyes by laccases - A mini-review. Nova
Biotechnologica et Chimica 15: 90-106.
46. Chivukula M, Renganathan V (1995)
Phenolic azo dye oxidation by laccase from Pyricularia
oryzae. Appl Environ Microbiol 61: 4374-4377.
47. Telke AA, Ghodake GS, Kalyani DC, Dhanve
RS, Govindwar SP (2011) Biochemical characteristics of a textile dye degrading
extracellular laccase from a Bacillus sp. ADR. Bioresour Technol 102:
1752-1756.
48. Chen H (2006) Recent advances in
azo dye degrading enzyme research. Curr Protein Peptide Sci 7: 101-111.
49. Telke AA, Kadam AA, Jagtap SS,
Jadhav JP, Govindwar SP (2010) Biochemical characterization and potential for
textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol Bioprocess Eng 15:
696-703.
50. Xingzu W, Cheng X, Dezhi S, Qi H
(2008) Biodecolorization and partial mineralization of Reactive Black 5 by a
strain of Rhodopseudomonas palustris.
J Environ Sci 20: 1218-1225.
51. Du LN, Li G, Zhao YH, Xu HK, Wang
Y, et al. (2015) Efficient metabolism of the azo dye methyl orange by Aeromonas sp. strain DH-6:
characteristics and partial mechanism. Int Biodeterioration Biodegradation 105:
66-72.
52. Afreen S, Fatma T (2013) Laccase
production and simultaneous decolorization of synthetic dyes by cyanobacteria.
Int J Innov Res Sci Eng Technol 2: 3563-3568.
53. Patel H, Gupte S, Gahlout M, Gupte
A (2014) Purification and characterization of an extracellular laccase from
solid-state culture of Pleurotus
ostreatus HP-1. 3 Biotech 4: 77-84.
54. Mongkolthanaruk W, Tongbopit S,
Bhoonobtong A (2012) Independent behavior of bacterial laccases to inducers and
metal ions during production and activity. Afr J Biotechnol 11: 9391-9398.
55. Afreen S, Bano F, Ahmad N, Fatma T
(2017) Screening and optimization of laccase from cyanobacteria with its
potential in decolorization of anthraquinonic dye Remazol Brilliant Blue R.
Biocatalysis Agric Biotechnol 10: 403-410.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Astronomy and Space Research
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)