1088
Views & Citations88
Likes & Shares
Northeastern India with its diverse topography ranging from plains of
Assam and Tripura to mountainous regions of Sikkim and Arunachal Pradesh
possesses great diversity of flora and fauna, including varieties of Capsicum
species. Although, Northeastern India is rich in genetic diversity of C. species, information about biotic and
abiotic stresses and the source of resistance, breeding approaches to improve
the chilli landraces of NE India, ethno-pharmacological applications,
capsaicin/capsaicinoid content and other useful compounds of many landraces are
still largely unexplored. This review article highlights about the various
research work carried out so far in chillies of NE India, related to genetic
diversity study and tissue culture techniques, etc.
Keywords: Capsicum species,
Northeast India, Genetic diversity, Landraces, Capsaicin
Abbreviations: NE India:
Northeast India; C. species: Capsicum species; CAU: Central Agricultural
University
INTRODUCTION
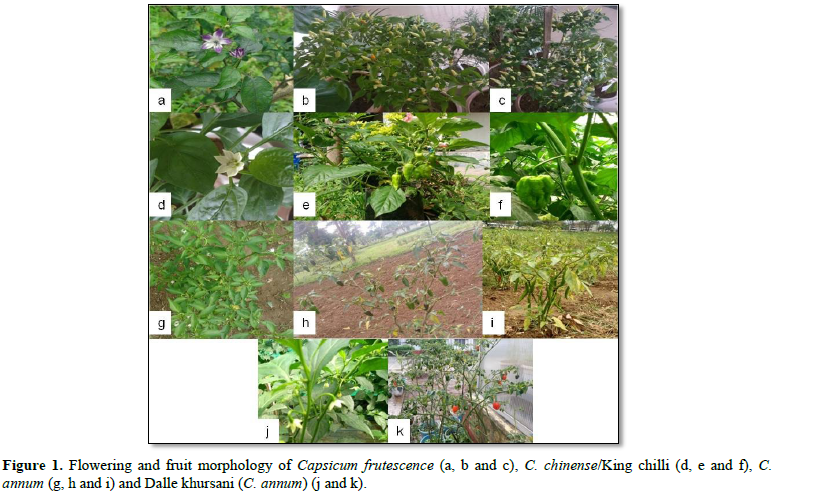
Chillies belong to the genus Capsicum of the Solanaceae family. Capsicum is derived from the Greek word ‘kapsimo’, meaning ‘to bite’ [1]. There are 25-30 species of Capsicum of which 5 species, i.e., C. annum L., C. frutescens mill, C. chinense, C. baccatum L. and C. pubescens have been domesticated and cultivated [2]. Capsicum species are diploids with most of them having 24 chromosome (n=x=12), but species with genome size of 2n=2x=32 [3] and 2n=2x=48 [4] also have been reported. It is a self-pollinated dicot plant. However, occurrence of cross pollination leads to the formation of variants within the species. The primary centre of origin of chilli is thought to be Mexico (American tropics) with secondary centers in Guatemala and Bulgaria [5]. Chilli is believed to have been introduced to India by Portuguese explorers [1] and to NE India by Christian missionaries [6]. The NE India has a rich natural heterogeneity due to its distinctive geographical location which has been identified as one of the twelve “Genetic Epicentres” for the evolution of world flora. In NE India, several Capsicum landraces are traditionally grown, which are distinguished based on their morphological appearance (Figure 1), flavor and pungency levels. These are referred to by a number of vernacular names, e.g. Naga King chilli (Bhut Jolokia/Bih Jolokia/Naga Jolokia, Lota Bhut, Dhan Jolokia, Krishna Jolokia, Mem Jolokia, Raja mircha, U-morok), In Sikkim, they are known as Dalle Khursani (round chillies), Thadey Khursani (erect fruit), Thalo Khursani (vegetable type), Jeerey Khursani (thin fruit), Lamchey Khursani (medium size-less pungent) and so on.
AREA AND PRODUCTION OF CHILLI IN NE STATES OF INDIA
India is the largest producer, consumer and exporter of chillies in the world [11]. In India, per capita consumption is highest for chillies, among the spices produced. It occupies around 30 % of the area shared among major spice crops of the country [12]. Though chillies are grown all over India, NE states contribute 51.72% of its annual production while having only 8% area under chilli cultivation (Spice Statistics, Spice Board, 2004). This region comprising the states of Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Sikkim and Tripura is located between 22-29.3° N and 89.7-97.8° E. Among the NE states, Assam has the maximum area under chilli cultivation, and it seems Nagaland is at top among the NE states in terms of production (Table 1). NE region of India is an excellent place to find the diverse range of chilli genotypes, because there are numerous heterogeneous landraces of chilli available in this region and these landraces serve as a reservoir of genetic variability for chilli breeders [13].
REPORTS ON ASSESSMENT OF GENETIC DIVERSITY IN CAPSICUM SPECIES OF NE INDIA
For strategic germplasm collection, maintenance, conservation and utilization, sound knowledge of diversity in any plant species is an important requirement [14]. Numerous researches have been conducted on genetic diversity of Capsicum using morphological as well as molecular markers. However, there has been very little research on genetic diversity of Capsicum accessions collected from NE India.
Mohammadi and Prasanna [14] assessed genetic diversity using morphological characters of 52 genotypes of Capsicum which were collected from different regions of NE India. Wide variations were observed in various morphological characters studied which suggested the diverse nature of germplasm collected. The study on genetic relationship of nineteen genotypes belonging to C. annuum, C. frutescence, C. chinense and Bhut Jolokia showed that Bhut Jolokia shared more markers from C. chinense than with C. frutescence. The average genetic similarity of Bhut Jolokia and C. chinense was 0.79 which is closer to the mean genetic diversity within C. chinense (0.82) than that within C. frutescens (0.85). Based on the presence of both C. frutescens and C. chinense specific markers in Bhut Jolokia, the study concluded it as an interspecific hybrid between the two species [15]. This study also identified some of the species specific markers which will be useful in species identification as well as for marker assisted breeding of chilli crops. Evaluation of genetic diversity of Naga Jolokia with morphological characters revealed a considerable amount of genetic variability with respect to fruit shape and color in this germplasm [16]. Twenty five chilli genotypes which were from NE region of India, evaluated for phytochemical composition and antioxidant potential showed variations for capsaicin, oleoresin, phenolics, carotenoids and other antioxidants contents [17]. Genetic differences between Bhut Jolokia, C. frutescens and C. chinense was revealed when six genotypes of Bhut Jolokia with eight known Capsicum species were characterized by using three nuclear genes, namely; ITS 1, ITS 2 and 5.8 S. However, the study failed to discriminate between C. frutescens and C. chinense [7]. Cluster analysis of genetic diversity of seven Capsicum landraces of C. annuum, C. frutescence and C. chinense using 10 RAPD showed two major clusters where C. frutescens and C. chinense genotypes grouped in one cluster apart from the C. annuum genotypes. The average genetic diversity was more in C. frutescens and C. chinense genotypes than in C. annuum [18]. The genetic diversity analysis of 53 chilli landraces of NE India using pun 1 locus revealed 79 SNPs and 3 indels in pun1 gene and when SSR markers and morphological traits were used to study diversity, they detected 3-9 alleles for each SSR locus with an average of 5.36 alleles/locus. Cluster analysis based on morphological and SSR markers separated the accessions with campanulate fruits type from those with erect fruits type [19]. When SSR and random amplified microsatellite polymorphism (RAMPO) markers were used to evaluate the genetic diversity of germplasm collected from nine countries which included 4 species of Capsicum, improved lines and 5 landraces from NE India, the dendrogram based on both the markers showed two major clusters and was able to separate C. annuum genotypes from others. The study revealed the presence of higher genetic diversity in other Capsicum spp. than C. annuum genotypes [20]. Genetic diversity based on twelve quantitative characters of 30 chilli (Capsicum annuum L.) genotypes collected from different regions of India, majority from Arunachal Pradesh, showed considerable variability among the genotypes for the character studied and some genotypes were identified as better parents for an efficient hybridization programme of chilli [21]. Protein profiling of 30 genotypes of chilli collected from NE India showed considerable variation in banding pattern of total protein which ranged from 7-19 numbers of bands [22]. Five different ecotypes of Bhut Jolokia collected from different locations of NE India showed significant difference in terms of phenolic content combined with ascorbic acid in the fruits [23]. Diversity analysis by estimating the bioactive compounds present in 72 chilli landraces of NE India showed significant difference in total phenolic and flavonoid content. The result also suggested that Capsicum frutescens L. is a potent source of natural antioxidants [13]. Significant intra and inter-specific variations for fruit morphological traits, fruiting habits and 65 fruit metabolites were observed in the collected Capsicum germplasm belonging to three Capsicum species, i.e., C. chinense (Bhut jolokia, 63 accessions), C. frutescens (17 accessions) and C. annuum (56 accessions). Phenotypic diversity of 72 landraces of chilli consisting predominantly of C. frutescence collected from different regions of NE India using 29 qualitative traits and 14 quantitative traits revealed the vast intra-specific diversity of C. frutescence landraces of NE India [24]. SDS-PAGE analysis of 31 chilli genotypes collected from different regions of NE India showed considerable variation in number of protein bands ranging from 8-18 and a total of 70 protein bands as per Rm values were identified by silver staining [25]. Study on about 30 Bhut Jolokia genotypes to determine the distinctiveness of Bhut Jolokia with the germplasm using SSR markers revealed 100% polymorphism in 9 out of 10 markers [26].
OTHER RESEARCH ACTIVITIES ON CHILLIES OF NE INDIA
Although NE India is rich in genetic diversity of chilli crops, there has been very little research conducted in chilli crops belonging to NE India regions. Therefore, there is a high probability of discovering a number of genes/alleles contributing to resistance against abiotic and biotic stresses, some chemicals/compounds that can be used as medicine and other secondary metabolites that are of high value. Below are some of the researches conducted on chillies of NE India for various purposes. Capsaicin and Capsaicinoid have been estimated from different chilli cultivars of NE India [27-30]. Growing environment, sowing time and crop geometry influenced the synthesis of capsaicin or capsaicinoids in Chilli pepper [31,32]. Comparative expression analysis (through qRT-PCR) of candidate genes involved in capsaicin/capsaicinoid biosynthesis pathway showed many fold higher expression of majority of the genes in C. chinense compared to C. frutescens and C. annuum suggesting that the possible reason for extremely high pungency might be due to the higher level of candidate gene(s) expression [33]. Many components of chilli fruits are known to have medicinal values. The ethno- pharmacological applications of king chilli and its derivatives is well described [34-36]. Susceptibility of the chilli genotypes to a number of abiotic and biotic stresses has restricted their potential yield. Abiotic stresses which significantly reduced the yield and quality of peppers include extreme of temperature, moisture, light, nutrients and pH among others. Biotic factors include susceptibility of chilli plants to various fungi, bacteria, viruses, nematodes and various insects. Among the biotic factors, the one that poses a great threat to cultivated chilli is viral infection [37]. All these problems can be overcome by identifying novel sources of resistance [38-40], heterosis breeding [41] and proper identification and management of causal organism [42-46]. Number of characteristics like very low germination percentage, recalcitrant nature, genotype dependent, etc. associated with the Capsicum creates problems in improvement of chilli plants by conventional breeding techniques as compared to other solanaceous crops [2]. Hence, the plant biotechniques which include plant tissue culture and genetic engineering are gradually becoming a functional aspect of classical breeding programs and boost the improvement in Capsicum crop. Many researchers [4,23,28,32,47-57] reported the efficient method for in vitro regeneration/micro-propagation of chillies belonging to NE India, that will help in further improvement of the chilli crop through biotechnological approaches that involve tissue culture.
CONCLUSION
The NE India has a rich natural heterogeneity due to its distinctive geographical location where several landraces of crops including Capsicum species are traditionally grown. These landraces of chillies have various traditional uses including uses in different ethnic cuisines and ethno-pharmacological use [58]. Although many studies have been done in chilli landraces of NE India including diversity/evolution, in vitro regeneration, scientific cultivation, diseases and their management, the potentiality of chilli landraces of NE India as the source of biotic and abiotic stress resistance, medicinal properties, etc. are still largely unexplored. Chilli cultivation in NE India also faces the problems like lack of warehouse/godown for proper storage, poor transport facility for harvested product and lack of market information to farmers for efficient and timely production of chilli on their farms. There is a need to conduct more researches on NE India chilli to develop varieties that are better yielding, tolerant to various stresses, etc. Further, awareness about market situation, cultivation techniques, proper storage and transportation will make chilli cultivation more remunerative for chilli farmers in NE India [59-61].
ACKNOWLEDGEMENT
We thank Dean, College of Post-Graduate Studies and Chairman, School of Crop Improvement, CAU (Imphal) for support. Bhutia KL and Bhutia ND are grateful to UGC, Government of India for National Fellowship.
1. Basu SK, De AK (2003) Capsicum: Historical
and botanical perspectives. In: De AK (ed.) The genus Capsicum. Taylor &
Francis, London, pp 1-15.
2. Kothari SL, Joshi A, Kachhawaha S,
Ochoa-Alejo N (2010) Chilli pepper - A review on tissue culture and
transgenics. Biotechnol Adv 28: 35-48.
3. Wang D, Bosland PW (2006) The genes of
Capsicum. Horticult Sci 41: 1169-1187.
4. Dafadar A, Das A, Bandopadhyay B, Jha TB
(2012) In vitro propagation and
molecular evaluation of Capsicum annuum
L. cultivar with high chromosome number (2n=48). Scientia Horticulturae 140:
119-124.
5. Salvador MH (2002) Genetic resources of
chilli (Capsicum spp.) in Mexico.
Proceedings of the 16th International Pepper Conference, Tampico,
Tamaulipas, Mexico, pp: 10-12.
6. Dhaliwal MS (2007) Solanaceous vegetables.
Handbook of vegetable crops. Kalyani Publishers, Ludhiana, pp: 34-76.
7. Purkayastha J, Alam SI, Gogoi HK, Singh L
(2012) Capsicum assamicum sp. nov.
(Solanaceae), from Assam, northeastern India. Ozean J Appl Sci 5: 55-66.
8. Simonne AH, Simonne EH, Eitenmiller RR, Mills
HA, Green NR (1997) Ascorbic acid and provitamin A contents in unusually
colored bell peppers (Capsicum annuum
L.). J Food Composition Anal 10: 299-311.
9. Reyes-Escogido MDL, Gonzalez-Mondragon EG,
Vazquez-Tzompantzi E (2011) Chemical and pharmacological aspects of capsaicin.
Molecules 16: 1253-1270.
10. Ravishankar GA, Suresh B, Giridhar P, Rao SR,
Johnson TS (2003) Biotechnological studies on Capsicum metabolite production
and plant improvement. In: De AK, editor. Capsicum: The genus Capsicum. London:
CRC Press.
11. http://www.fao.org/faostat/en/#rankings/countries_by_commodity/
12. Indian Horticulture Database (2011) National
Horticulture Board. New Delhi: Aristo Printing Press, pp: 6-7.
13. Dutta SK, Singh SB, Saha S, Akoijam RS,
Boopathi T, et al. (2017a) Diversity in bird’s eye chilli (Capsicum frutescens L.)
landraces of north-east India in terms of antioxidant activities. Proc Natl
Acad Sci India Sect B Biol Sci 87: 1317.
14. Mohammadi SA, Prasanna BM (2003) Analysis of
genetic diversity in crop plants - Salient statistical tools and
considerations. Crop Sci 43: 1235-1248.
15. Bosland PW, Baral JB (2007) BhutJolokia - The
world’s hottest known chilli pepper is a putative naturally occurring
interspecific hybrid. Horticult Sci 42: 222-224.
16. Bhagowati RR, Changkija S (2009) Genetic
variability and traditional practices in Naga King Chili landraces of Nagaland.
Asian Agri History 13: 171-180.
17. Dubey RK, Singh V, Upadhyay G, Pandey AK
(2015) Assessment of phytochemical composition and antioxidant potential in
some indigenous chilli genotypes from northeast India. Food Chem 188: 119-125.
18. Sanatombi K, Sen-Mandi S, Sharma GJ (2010)
DNA profiling of Capsicum landraces of Manipur. Scientia Horticulturae 124:
405-408.
19. Yumnam JS, Tyagi W, Pandey A, Ng TM, Rai M
(2012) Evaluation of genetic diversity of chilli landraces from north eastern
India based on morphology, SSR markers and the Pun1 Locus. Plant Mol Biol Rep
30: 1470-1479.
20. Rai RP, Glint VD, Babu KN (2013) In vitro plant regeneration in Capsicum chinense Jacq. (Naga Chili). J
Appl Biol Biotechnol 3: 30-33.
21. Yatung T, Dubey RK, Singh V, Upadhyay G
(2014a) Genetic diversity of chilli (Capsicum
annuum L.) genotypes of India based on morphochemical traits. Aust J Crop
Sci 8: 97-102.
22. Yatung T, Dubey RK, Singh V, Upadhyay G,
Singh S (2014b) Studies on seed protein profiling in chilli (Capsicum annuum L.) genotypes of
northeast India. Aust J Crop Sci 8: 369-377.
23. Kundu S, Das A, Haque SM, Ghosh B (2015)
Chemotypic diversity in different Bhut Jolokia fruits: In vitro conservation and mass propagation of superior ecotype. Int
J Pharm Sci Rev Res 34: 47-53.
24. Dutta S, Singh SB, Vanlalhmangaiha, Banerjee
A, Akoijam RS, et al. (2017b) Capsicum
frutescens L. landraces of northeast India: from phenotypic diversity
perspective of unexplored collection. Proc Natl Acad Sci India Sect B Biol Sci,
pp: 1-12.
25. Alice AK, Dubey RK, Pandey AK, Singh V, Singh
S (2017) Studies on genetic diversity in chilli (Capsicum annuum L.) through SDS-PAGE protein profiling. Int J Chem
Stud 5: 465-470.
26. Adluri PK, Borah AR, Nath PD (2017a) Study of
genetic diversity of Bhut Jolokia germplasm in north east India by SSR markers
and morphology. Int J Pure Appl Biosci 5: 1657-1665.
27. Bharathi LK, Rengasamy S, Singh R, Prabhu KV,
Sharma A, et al. (2011). Estimation of capsaicin and capsaicinoid contents of
high pungent chilli accessions of Andaman and Nicobar Islands and northeast
India. Indian J Horticult 68: 551-555.
28. Kehie M, Kumaria S, Tandon P (2013) In vitro plantlet regeneration from
cotyledon segments of Capsicum chinense
Jacq. cv. Naga King Chili and determination of capsaicin content in fruits of in vitro propagated plants by high
performance liquid chromatography. Scientia Horticulturae 164: 1-8.
29. Sweat KG, Broatch J, Borror C, Hagan K,
Cahill TM (2016) Variability in capsaicinoid content and scoville heat ratings
of commercially grown Jalapeño, Habanero and Bhut Jolokia peppers. Food Chem
210: 606-612.
30. Ananthan R, Subhash K, Longvah T (2018)
Capsaicinoids, amino acid and fatty acid profiles in different fruit components
of the world hottest Naga king chilli (Capsicum
chinense Jacq.). Food Chem 238: 51-57.
31. Moirangthem SS, Gogoi S, Thongbam PD, Ramya
KT, Fiyaz RA, et al. (2014). Effect of sowing time and crop geometry on the
Capsaicinoid content in Bhoot Jolokia (Capsicum
chinense Jacq.). Food Sci Technol 51: 1974-1981
32. Bhutia KL, Ng TM, Khanna VK (2018) In vitro direct regeneration of Dalle
Khursani (Capsicum annum) from
salicylic acid treated explants. J Pharmacogn Phytochem 7: 1008-1012.
33. Sarpras M, Gaur R, Sharma V, Chhapekar SS,
Das J, et al. (2016) Comparative analysis of fruit metabolites and pungency
candidate genes expression between Bhut Jolokia and other Capsicum species.
PLoS One 11: e0167791.
34. Meghvansi MK, Siddiqui S, Khan MH, Gupta VK,
Vairale MG, et al. (2010). Naga chilli: A potential source of capsaicinoids
with broad-spectrum ethnopharmacological applications. J Ethnopharmacol 132:
1-14.
35. Liu Y, Nair MG (2010) Capsaicinoids in the
hottest pepper Bhut Jolokia and its antioxidant and anti-inflammatory
activities. Nat Prod Commun 5: 91-94.
36. Baruah S, Zaman MK, Rajbongshi P, Das S
(2014) A review on recent researches on Bhut Jolokia and pharmacological
activity of capsaicin. Int J Pharm Sci Rev Res 24: 89-94.
37. Venkataiah P, Subhash K (2003) Research
articles: Thidiazuron induced high frequency adventitious shoot formation and
plant regeneration in Capsicum annuum
L. J Plant Biotechnol 5: 245-250.
38. Pandey U, Pandey J (2002) Anti-bacterial
properties of cyanobacteria: A cost-effective and eco-friendly approach to
control bacterial leaf spot disease of chilli. Curr Sci 82: 262-264.
39. Garg R, Kumar S, Kumar R, Loganathan M, Saha
S, et al. (2013). Novel source of resistance and differential reactions on
chilli fruit infected by Colletotrichum
capsici. Australasian Plant Pathol 42: 227.
40. Adluri PK, Baldoldiya GM, Nath PD (2017b)
Screening of Bhut Jolokia (Capsicum
chinense Jacq.) germplasm of northeast India against chilli leaf curl
virus. Int J Pure Appl Biosci 5: 1189-1196.
41. Bhutia ND, Seth T, Shende VD, Dutta S,
Chattopadhyay A (2015) Estimation of heterosis, dominance effect and genetic
control of fresh fruit yield, quality and leaf curl disease severity traits of
chilli pepper (Capsicum annuum L.).
Scientia Horticulturae 182: 47-55.
42. Rajapakshe RGAS, Ranasinghe JADAR (2002)
Development of variety screening method for anthracnose disease of chilli (Capsicum annuum L.) under field
conditions. Trop Agric Res Extension 5: 7-11.
43. Kumar R, Barman A, Jha G, Ray S (2013)
Identification and establishment of genomic identity of Ralstonia solanacearum isolated from a wilted chilli plant at
Tezpur, northeast India. Curr Sci 105: 1571-1578.
44. Banerjee A, Dutta R, Roy S, Ngachan SV (2014)
First report of chilli veinal mottle virus in Naga chilli (Capsicum chinense) in Meghalaya, India. Virus Dis 25: 142-143.
45. Pandey KK, Gupta RC (2015) Management of
anthracnose (Colletotrichum capsici)
in chilli (Capsicum annum L.) through
fungicides, bioagents and hand picking methods. J Spices Aromatic Crops 24:
141-144.
46. Ng TC, Singh YH, Sumitra PH, Singh S, Singh
SR, et al. (2017). Molecular based indexing of viral disease complex of king
chilli (Capsicum chinense J.) in
north eastern region of India. J Pharmacogn Phytochem 6: 2004-2008.
47. Sanatombi K, Sharma GJ (2006) In vitro regeneration and mass
multiplication of Capsicum annuum L.
J Food Agric Environ 4: 205-208.
48. Sanatombi K, Sharma GJ (2007.
Micropropagation of Capsicum frutescens
L. using axillary shoot explants. Scientia Horticulturae 113: 96-99.
49. Sanatombi K, Sharma GJ (2008a) In vitro propagation of Capsicum chinense Jacq. Biologia
Plantarum 52: 517-520.
50. Sanatombi K, Sharma GJ (2008b) In vitro plant regeneration in six
cultivars of Capsicum spp. using
different explants. Biologia Plantarum 52: 141-145.
51. Sanatombi K, Sharma GJ (2012) In vitro regeneration of Capsicum chinense Jacq. Curr Trends
Biotechnol Pharm 6: 66-72.
52. Kehie M, Kumaria S, Tandon P (2012) Osmotic
stress induced-capsaicin production in suspension cultures of Capsicum chinense Jacq. cv. Naga king
chili. Acta Physiol Plant 34: 2039.
53. Gogoi S, Acharjee S, Devi J (2014) In vitro plantlet regeneration of Capsicum chinense Jacq. cv. ‘Bhut
jalakia’: Hottest chili of northeastern India. In Vitro Cell Dev Biol Plant 50:
235.
54. Bora G, Gogoi HK, Handique PJ (2014) Effect
of silver nitrate and gibberellic acid on in
vitro regeneration, flower induction and fruit development in Naga chilli.
Asia Pac J Mol Biol Biotechnol 22: 137-144.
55. Mangang RKJ (2014) In vitro callus induction of placental tissues of Capsicum chinense Jacq. cv. ‘umorok’ using
different concentrations and combinations of growth hormones. Int J Interdiscip
Multidiscip Stud 1: 63-66.
56. Gayathri N, Gopalakrishnan M, Sekar T (2015) In vitro micropropagation of Capsicum chinense Jacq. (Naga king
chili). Asian J Plant Sci Res 5: 13-18.
57. Bhutia KL, Ng TM, Khanna VK (2016) In vitro regeneration of Dalle Khursani,
an important chilli cultivar of Sikkim, using various explants. Agrotechnology
5: 142.
58. Singh A, Singh RK, Sureja A (2007) Cultural
significance and diversities of ethnic foods of northeast India. Indian Journal
of Traditional Knowledge 6: 79-94.
59. Horticulture at a Glance (2017) Government of
India, Ministry of Agriculture and Farmers Welfare, Horticulture Statistics
Division
60. Sharma A (2016) Sustainable economic analysis
and constraints faced by the Naga king chilli growers in Nagaland. Indian J
Agric Res 50: 220-225.
61. http://www.indianspices.com/html/s0623chl.htm
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)