972
Views & Citations10
Likes & Shares
Pancreatic cancer is considered as a chemoresistant tumor because of its
abundant cancer stroma which contains cancer-associated fibroblasts (CAFs).
Nab-paclitaxel (Nab-PTX, cytoskeletal anticancer drug) is expected as a key
drug for pancreatic cancer. The patients of pancreatic cancer treated with
Nab-Paclitaxel show remarkable tumor reduction in size. We analyzed total 20
surgically resected cases of pancreatic ductal adenocarcinoma; ten cases
treated with Nab-PTX before surgical operation (treated group) and ten cases
without neoadjuvant chemotherapy (untreated group). The treated group showed
high density of aniline blue (AnB) positive collagen bundles in the stroma and
low density of α-smooth muscle actin (α-SMA) positive CAFs. On the other hand,
the untreated group exhibited low density of AnB positive collagen bundles and
high density of CAFs. We speculated that the Nab-PTX neoadjuvant chemotherapy
plays in roles of stromal collagenous fibrosis and inactivation of CAFs.
Keywords: Pancreatic ductal
adenocarcinoma, Neoadjuvant chemotherapy, Nab-Paclitaxel, Cancer associated
fibroblast
INTRODUCTION
In this study we
investigated why Nab-PTX has prominent anticancer effects, analyzing histopathological
specimens of pancreatic cancer stroma which were performed neoadjuvant
chemotherapy with Nab-PTX.
MATERIALS AND METHODS
We investigated total 20 surgically resected
cases of pancreatic ductal adenocarcinomas; ten cases treated with Nab-PTX
before surgical operation (treated group) and ten cases without neoadjuvant
chemotherapy (untreated group). Numbers of the cases were limited because the
Nab-PTX treatment has recently established as the neoadjuvant chemotherapy for
pancreatic cancer.
We evaluated the cancer stromal phenotypes
using Heidenhain’s AZAN trichrome stain (AZAN stain) [10,11] and
immunohistochemical α-smooth muscle actin (α-SMA) stain. Collagen bundles in
the cancer stroma are stained with aniline blue (AnB) of AZAN stain, while CAFs
in the cancer stroma are
immunohistochemically positive for α-SMA. We
RESULTS
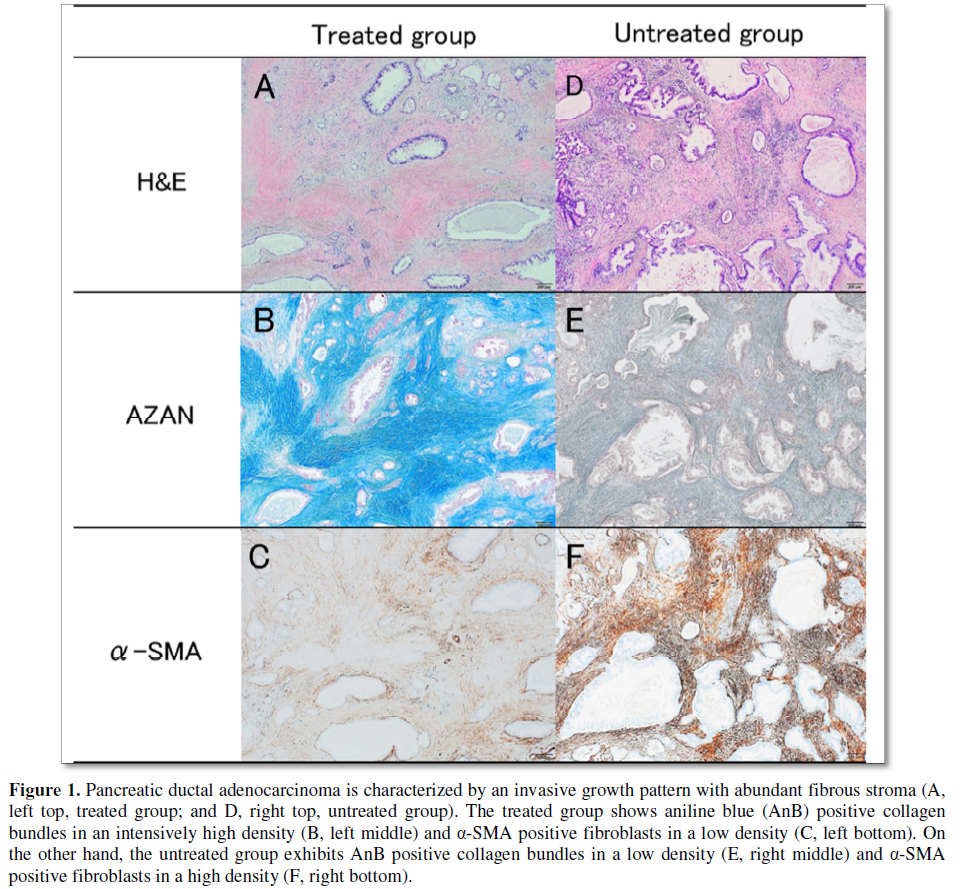
Pancreatic ductal
adenocarcinoma was characterized by an invasive growth pattern with abundant
fibrous stroma in both treated/untreated groups (Figure 1). However, the treated/untreated groups revealed
different distribution pattern of AnB positive collagen bundles and α-SMA
positive CAFs. The treated group showed the very high density of collagen
bundles and the low density of CAFs. On the other hand, the untreated group
exhibited the low density of collagen bundles and high density of CAFs.
Table 1 is the results of Image J analyzation. Student t-test
proved that the treated group significantly increased AnB positive collagen
bundle area (P<0.01) and significantly decreased α-SMA positive CAF area
(P<0.01).
DISCUSSION AND CONCLUSION
In the present study,
we demonstrated the Nab-PTX neoadjuvant chemotherapy induced the unique fibrous
stroma of pancreatic ductal adenocarcinoma, i.e., dense AnB positive fibrous
stroma with limited numbers of CAFs. We think the dense collagenous fibrosis in
the stroma contributes to tumor shrinkage in an anticancer effective manner. We
have speculated that the pancreatic ductal adenocarcinomas in the untreated
group continuously induced active CAFs in the stroma, and made use of the CAFs
for the chemoresistance [5,6]. However, the treated group with Nab-PTX rapidly
induced AnB positive collagen bundles in the stroma, and then decreased numbers
of CAFs.
Our previous studies
reported that the transcriptional factors associated epithelial-mesenchymal
transition (EMT) contributed pancreatic cancer invasiveness/aggressiveness, and
were thought to be related with CAFs [12,13]. The Nab-PTX neoadjuvant
chemotherapy plays in roles of stromal collagenous fibrosis and inactivation of
CAFs. However, details of molecular mechanisms of Nab-PTX are still unknown. We
try to analyze the molecular mechanisms of collagenous fibrosis as the Nab-PTX
anticancer effects in the near future.
1.
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer
statistics. CA Cancer J Clin 60: 277-300.
2.
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO
classification of tumors of the digestive system. 4th Edn. International Agency
for Research on Cancer (IARC).
3.
Tsutsumi S, Morohashi S, Kudo Y, Akasaka H, Ogasawara
H, et al. (2011) L1 cell adhesion molecule (L1CAM) expression at the cancer
invasive front is a novel prognostic marker of pancreatic ductal
adenocarcinoma. J Surg Oncol 103: 669-673.
4.
Sakuraba S, Morohashi S, Yoshizawa T, Tsutsumi S,
Kimura N, et al. (2015) MUC5AC-negative phenotype is correlated with poor
patient prognosis of pancreas head ductal carcinoma. Hirosaki Med J 66: 28-37.
5.
McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, et
al. (2014) Role of pancreatic stellate cells in chemoresistance in pancreatic
cancer. Front Physiol 5: 141.
6.
Schober M, Jesenofsky R, Faissner R, Weidenauer C,
Hagmann W, et al. (2014) Desmoplasia and chemoresistance in pancreatic cancer.
Cancers (Basel) 6: 2137-2154.
7.
Zalatnai A, Molnar J (2007) Molecular background of
chemoresistance in pancreatic cancer. In Vivo 21: 339-347.
8.
Miyashita T, Tajima H, Makino I, Okazaki M, Yamaguchi
T, et al. (2018) Neoadjuvant chemotherapy with gemcitabine plus Nab-paclitaxel
reduces the number of cancer-associated fibroblasts through depletion of pancreatic
stroma. Anticancer Res 38: 337-343.
9.
Alvarez R1, Musteanu M, Garcia-Garcia E, Lopez-Casas
PP, Megias D, et al. (2013) Stromal disrupting effects of nab-paclitaxel in
pancreatic cancer. Br J Cancer 109: 926-933.
10.
Ohno S, Tachibana M, Fujii T, Ueda S, Kubota H, et al.
(2002) Role of stromal collagen in immunomodulation and prognosis of advanced
gastric carcinoma. Int J Cancer 97: 770-774.
11.
Heidenhain M (1915) Uber die Mallorysche
Bindegewebsf¨arbung mit Karmin und Azokarmin als Vorfarben. Z Wissenschaft
Mikrosk Mikrosk Techn 32: 361-372.
12.
Wu Y, Kijima H (2018). BHLH transcription factors DEC1
and DEC2: From structure to various diseases. Biomed Res J 2: 28-33.
13.
Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, et al.
(2012) The BHLH transcription factor DEC1 plays an important role in the
epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol 41:
1337-1346.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Pathology and Toxicology Research
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Allergy Research (ISSN:2642-326X)