788
Views & Citations10
Likes & Shares
The present study was carried out to investigate inhibition of proteases extracted from the leaves of fennel, parsley and lemongrass in the presence of selected heavy metals. The activity in decreasing order for the three samples, lemongrass, fennel and parsley was 0.341 units/ml, 0.330 units/ml and 0.176 units/ml. The heavy metals selected were Fe, Cu, Cr, Co, Ag and Hg. Standard stock solutions for these heavy metals were prepared and protease inhibition studies were carried out using the principle of casein-Coomassie dye-binding. The greatest inhibition occurred on lemongrass protease and both Hg and Co had reduced activity considerably at minimum concentrations of 40 mg/L and 30 mg/L to 66.3% and 64%, respectively. EDTA increased lemongrass protease activity to 0.458 and 0.602 units/ml at 10 mM and 1 mM concentrations. Optimization of protease inhibition studies for 30 mg/L of Co and 40 mg/L of Hg was done; and then sensitivity of the enzyme to detect these heavy metals in wastewater samples was evaluated at 37°C (60 min incubation time) for cobalt and at 35°C (20 min incubation time) for mercury. The crude lemongrass protease could detect Co and Hg at minimum concentrations of 7.21 mg/L and 2.35 mg/L, respectively in environmental samples. These values were also assayed using AAS for confirmation. The data analysis showed that lemongrass protease can be used as a potential biomonitoring tool for detecting elevated toxicity levels of mercury and cobalt in wastewater samples.
INTRODUCTION
Enzymatic bioassays offer an inexpensive, reliable alternative for detecting and quantifying heavy metals with comparable selectivity and sensitivity. These are desirable because they are simple, require no complex instruments and hence, people can use them easily for preliminary analysis [11]. Recent works have focused on exploiting proteases for such bioassays because of their remarkable stability over a broad range of temperature and pH. These enzymes are primarily involved in the hydrolysis of peptide bonds in proteins to degrade them. Apart from their physiologic importance, these also play an integral role in industries and occupy 60% of the overall global demand in enzyme market [12]. Promising results were obtained for plant proteases such as bromelain, papain; and proteases extracted from tomatoes, garlic and Coriandrum sativum for heavy metal detection [13-17]. Due to their applicability and economic significance, much research is currently being done on different plant protease classes and vast literature is available on their biotechnological aspects.
In this work, three plants, that are a household name in Asian cooking, were selected to investigate their level of protease activity and whether they could be promising candidates for developing an inhibitive enzyme assay for heavy metal detection. The plants selected for this purpose were fennel (Foeniculum vulgare), parsley (Petroselinum crispum) and lemongrass (Cymbopogon citratus) belonging to the genus Foeniculum, Petroselinum and Cymbopogon, respectively. The characterization of their proteases has not been cited in literature. To the best of our knowledge this is the first report on evaluation of the effect of selected heavy metals on these plant proteases.
MATERIALS AND METHODS
Reagent preparation
Preparation of buffers: 50 mM sodium and potassium phosphate buffers were prepared according to standard protocols. Sodium carbonate buffer was also prepared by mixing together calculated amounts of salts. Adjustment of buffer pH was made using 1 M NaOH and 1 M HCl [18].
Preparation of Bradford reagent: 0.1 g of Coomassie brilliant blue was measured, dissolved in 50 ml of 95% ethanol and 100 ml of 85% phosphoric acid, the final volume made up to 1000 ml with distilled water, stirred overnight in a shaking incubator at 30°C, filtered with Whatman filter paper 1 and stored in dark bottles until use [19].
Preparation of casein solution: 2 g of casein powder was dissolved in 100 ml distilled water and the pH was adjusted to 8.0 using 5 M NaOH and 5 M HCl. The solution was incubated overnight in a shaking incubator at 60°C, filtered through several layers of cheesecloth and stored in refrigerator for further use [17].
Preparation of heavy metals solutions: 1000 mg/L stock solutions of iron, chromium, silver, cobalt, mercury and copper were prepared in distilled water. Working solutions of 100 mg/L were prepared for each of the heavy metals and then 5 mg/L to 40 mg/L dilutions were prepared for inhibition studies.
Extraction of plant protease
Proteases from lemongrass, fennel and parsley were extracted using the modified method of Jiang et al. [20]. Plant leaves were taken, properly washed, chopped in fine pieces and placed in a falcon tube containing 0.5 mM sodium phosphate buffer. The falcon tube containing the chopped plant pieces was kept overnight at 4°C for two days following which the mixture was blended for 20 s and subjected to a 5 min cooling period. This cycle of blending and cooling was continued until a homogenized mix was obtained. The crude extract was kept in the refrigerator (4°C) to prevent damage. All falcon tubes were labeled accordingly. The same procedure was repeated for the three plants in triplicates.
Standard BSA and tyrosine assays were performed for each plant to estimate their protein content and enzyme activity [18].
Estimation of protease activity
Sigma’s casein assay was used to evaluate protease activity of the three samples [21]. 5 ml casein incubated in a water bath at 37°C for 5 min was mixed with 0.5 ml enzyme and incubated for ten minutes. Then 5 ml TCA reagent and 0.5 ml enzyme was added and test tubes were incubated for 30 min. Following incubation, the test tubes were filtered using filter paper and 2 ml was taken of the test tubes. 5 ml sodium carbonate solution and 1 ml Folin phenol reagent was added and the solution was incubated for 30 min. Experiment was performed in triplicates for all the three samples and absorbance was measured at 660 nm using UV spectrophotometer.
Protease inhibition studies
The samples which gave the greatest degree of inhibition were further investigated by making five mg/L dilutions (5 mg/L, 10 mg/L, 20 mg/L, 30 mg/L and 40 mg/L). Mercury and cobalt were the heavy metals which had reduced enzymatic activity of lemongrass considerably so these were investigated by making mg/L dilutions. The studies were performed in triplicates and means were calculated.
Effect of EDTA on protease activity
The effect of an enzyme inhibitor, EDTA, on lemongrass protease was studied by pre-incubating the enzyme mixture with EDTA at final EDTA concentrations of 1 and 10 mM for 10 min at 25°C and estimating the enzyme activity using casein assay [21].
Optimization of protease inhibition studies
To obtain optimum conditions for heavy metal detection, protease inhibition assay was optimized by varying temperature and incubation time. The assay was performed at temperatures 35°C, 37°C, 40°C, 45°C, 50°C, 55°C and 60°C and; incubation times ranging from 15-60 min.
Collection of environmental samples
Waste water samples were collected from industrial outlets (tanneries and chemical industries) and the major drains of River Ravi, i.e., Mehmood Booti drain, Hudiara drain, Babu Sabu drain, Sattu katla drain, Mian Mir drain, Farrukhabad drain, Shahdera drain (left bank), Munshi hospital drain, Taj company drain, Bakar mandi drain and minor drains such as Township municipal waste drain and Children’s hospital drain. Water was collected about 15-20 cm from the surface and placed in thoroughly washed bottles to which few drops of concentrated nitric acid were added.
Heavy metal determination
Digestion of samples: 10 ml of sample was mixed with a mixture of 5 ml conc. nitric acid and 5 ml conc. HCl, gently swirled and covered with watch glass for 1 h. Samples were then heated on a hot plate until the solution became clear, filtered using Whatman filter paper 1 and diluted up to 50 ml using distilled water [22].
Standard preparation: Standards of concentrations ranging from 5 mg/L to 50 mg/L were prepared from 1000 mg/L stock solutions of cobalt and mercury.
Instrumental analysis: The standards and digested samples were analyzed using Atomic Absorption Spectrophotometer (AAS) to determine heavy metal concentration.
Enzymatic bioassay
A modified method of Shukor et al. [15] was employed to test the efficiency of crude lemongrass protease in detecting mercury and cobalt in environmental samples. The assay was performed at predetermined optimized conditions, i.e., incubation time of 60 min and 37°C for cobalt; and 20 min of incubation time and 35°C for mercury. The bioassay was carried out in triplicates.
DATA ANALYSIS
Statistical analysis of all the data was done using Microsoft Excel 2013. All data are expressed as mean ± SEM. Moreover, the results obtained from AAS were compared with the results obtained from inhibitive protease assay to evaluate the efficacy of this bioassay.
RESULTS AND DISCUSSION
Screening of plant proteases
Protease from three plants, fennel, parsley and lemongrass was extracted. The protein content and enzyme activity of the three samples was calculated because it was important to find out which samples gave the greatest protease activity since, in order for them to be excellent choices to develop the inhibitory assay, sufficiently high protease activity is ideal [13]. The relative protein concentrations, after comparing with BSA standard, were found to be 0.38 mg/ml, 0.46 mg/ml and 0.34 mg/ml for fennel, lemongrass and parsley respectively. The protease activity determined by casein assay that uses casein as a substrate was found to be 0.330, 0.176 and 0.341 (units/ml) for fennel, parsley and lemongrass, respectively.
Protease inhibition studies
For parsley, it was seen that iron and mercury inhibited the proteolytic activity more as compared to other heavy metals. Iron reduced it to 38.9% and mercury to 43%. Copper and silver had the least inhibitory action and reduced the activity to only 57% and 55.2%, respectively. For lemongrass, mercury, cobalt and silver inhibited the activity to 11%, 12.9% and 34%, respectively. Chromium had the least effect and reduced the activity to 85.6% only. Iron and copper gave intermediate values in the range between 70-80%.
Effect of EDTA on protease activity
EDTA experiments with lemongrass protease showed that enzyme activity was enhanced by EDTA as it increased from 0.341 units/ml to 0.458 and 0.602 units/ml at 10 mM and 1 mM EDTA concentrations. The stimulated enzyme activity thereby indicated that the lemongrass protease is a non-metalloprotease, i.e., it lacks the presence of metals in its catalytic site. The structural rigidity of the protease [23] and the removal of traces of unknown metal ion inhibitors in the reaction mixture could have possibly contributed to the enhanced protease activity of lemongrass.
Optimization of protease inhibition studies
Since the lemongrass protease showed greatest inhibition at 40 mg/L of mercury and 30 mg/L of cobalt, so optimization of protease inhibition assay was done for these two concentrations of the respective heavy metals so as to determine the optimum temperature and incubation time at which the protease was inhibited to the maximum.
Protease inhibition by environmental samples
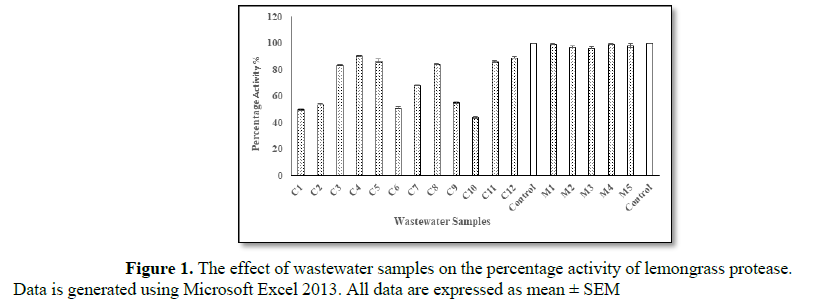
The presence or absence of heavy metals in various samples can be detected qualitatively by the blue or brown color of the solution after performing principal inhibition assay [15]. One aspect which is rather important is that the significant color changes with Bradford reagent will only be observable if the protease activity is sufficiently high [13]. Lemongrass had given the greatest protease activity of the three plants and to test the potential of the crude lemongrass protease in detecting mercury and cobalt in wastewater samples, twelve water samples for cobalt and five for mercury were collected from different sources in and around Lahore, Pakistan. These were selected because maximum inhibition of lemongrass protease was found at high concentrations, i.e., 30 mg/L of cobalt and 40 mg/L of mercury.
The results of inhibition assay were compared with those obtained from AAS for validation (Tables 3 and 4). The results from AAS showed that the samples were highly contaminated with cobalt and mercury, the values exceeding their maximum permissible limit, i.e., 0.001 mg/L and 0.05 mg/L for mercury and cobalt, respectively [24,25].
Lemongrass protease exhibited reduced sensitivity towards mercury as compared to proteases from tomato, Coriandrum sativum, garlic, papaya and pineapple [13-17]. However, estimation of mercury in wastewater samples demonstrated comparable values to those determined using AAS. Thus, illustrating a significant interrelationship between mechanical method and inhibition assay. Therefore, an inhibitive bioassay can be developed using lemongrass protease for routine detection of mercury and cobalt in environmental samples for the biomonitoring and control of ongoing heavy metal pollution.
CONCLUSION
The inhibition study based on the binding of casein with Coomassie dye conducted for the six heavy metals showed that both fennel and parsley gave intermediate inhibitions within the range of 40-70% while lemongrass, having the greatest activity, exhibited the highest inhibition as both mercury and cobalt had reduced its activity to 11% and 12.9% respectively at 1000 mg/L. Further analysis of the protease from lemongrass revealed its sensitivity to be at 30 mg/L of cobalt and 40 mg/L of mercury. This will be useful as an assay in regions that exhibit levels in this range or higher when compared to safe levels. Inhibition studies with wastewater samples at optimized parameters showed positive results that were further confirmed by AAS. Therefore, crude lemongrass protease, combined with its activity over a broad range of temperature, can be a potential candidate for detecting elevated levels of mercury and cobalt at a minimum concentration of 2.35 mg/L for mercury and 7.21 mg/L for cobalt in various environmental samples. Further characterization and purification will help in improving the sensitivity of lemongrass protease towards selected heavy metals.
ACKNOWLEDGEMENT
This project was supported by funds from Kinnaird College for Women, Lahore, Pakistan.
CONFLICT OF INTEREST STATEMENT
1. Afzal M, Shabir G, Iqbal S, Mustafa T, Khan QM, et al. (2014). Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. CLEAN Soil Air Water 42: 1133-1139.
2. Amin N, Ibrar D, Alam S (2014) Heavy metals accumulation in soil Irrigated with industrial effluents of Gadoon industrial estate, Pakistan and its comparison with fresh water Irrigated soil. J Agric Chem Environ 3: 80-87.
3. Ejaz N, Hashmi HN, Ghumman AR (2011) Water quality assessment of effluent receiving streams in Pakistan: A case study of Ravi River. Mehran Univ Res J Eng Technol 30.
4. Rehman W, Zeb A, Noor N, Nawaz M (2007) Heavy metal pollution assessment in various industries of Pakistan. Environ Geol 55: 353-358.
5. Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, et al. (2014)Pollution status of Pakistan: A retrospective review on heavy metal contamination of water, soil and vegetables. BioMed Res Int 2014: 1-29.
6. Jabeen G, Javed M, Azmat H (2012) Assessment of heavy metals in the fish collected from the River Ravi, Pakistan. Pak Vet J 32: 107-111.
7. Masdor NA, Said NAM (2011) Partial purification of crude stem bromelain improves its sensitivity as a protease inhibitive assay for heavy metals. Aust J Basic Appl Sci 5: 1295-1298.
8. Sahlani MZ, Halmi MIE, Masdor NA, Baskaran G, Wasoh H, et al. (2014) A rapid inhibitive assay for the determination of heavy metals using α-chymotrypsin; a serine protease. Nanobio BioNano 1: 41-46.
9. Turdean GL (2011) Design and development of biosensors for the detection of heavy metal toxicity. Int J Electrochem 2011.
10. Kurniawan TA, Chan GYS, Lo WH, Babel S (2006). Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118: 83-98.
11. Baskaran G, Kasim MHM, Salvamani S, Shukor MY (2014) Field trials on heavy metals using alpha-chymotryopsin enzyme assay. J Environ Microbiol Toxicol 2: 25-34.
12. Feijoo-Siota L, Villa TG (2010) Native and biotechnologically engineered plant proteases with industrial applications. Food Bioprocess Technol 4: 1066-1088.
13. Baskaran G, Masdor NA, Arif M, Shukor MY (2013) An inhibitive enzyme assay to detect mercury and zinc using protease from Coriandrum sativum. ScientificWorldJournal.
14. Halmi MIE, Sakeh NSM, Masdor NA, Baskaran G, Wasoh H, et al. (2014) The application of plant protease from garlic (Allium sativum) for biomonitoring of heavy metals in the environment. Asian J Plant Biol 2: 15-21.
15. Shukor Y, Baharom NA, Rahman FA, Abdullah MP, Shamaan NA, et al. (2006) Development of a heavy metals enzymatic-based assay using papain. Analytica Chimica Acta 566: 283-289.
16. Shukor MY, Masdor N, Baharom NA, Jamal JA, Abdullah MPA, et al. (2007) An inhibitive determination method for heavy metals using bromelain, a cysteine protease. Appl Biochem Biotechnol 144: 283-291.
17. Yunus M, Shukor Y (2013) An inhibitive determination method for heavy metals using tomato crude proteases. Asian J Plant Biol 1: 10-14.
18. Wiley A (2012). Current protocols essential laboratory techniques. 2nd Edn. (United States: John Wiley & Sons).
19. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
20. Jiang W, Zhou X, Zhao Y, Liu P (2002) Identification of a senescence-related protease in coriander leaves. Chin Sci Bull 47: 1096-1099.
21. Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay - casein as a substrate. J Vis Exp pii: 899.
22. Azeem HA (2009) Analysis of industrial waste water from Kot Lakhpat area (Lahore, Pakistan) by atomic absorption spectrometer. Biologia (Pakistan) 55: 35-41.
23. Amid M, Manap MYABD, Zohdi NK (2014) Purification and characterization of alkaline-thermostable protease enzyme from pitaya (Hylocereus polyrhizus) waste: A potential low cost of the enzyme. BioMed Res Int 2014: 259238.
24. Department of Environment (DOE) (2001) Environmental quality report. Department of Environment, Ministry of Science, Technology and the Environment, Malaysia.