30474
Views & Citations29474
Likes & Shares
Birnessite related layered manganese oxides are a versatile family of
compounds. Here we summarize the wide range of applications that these phases
have and the numerous synthesis strategies proposed for their production at the
nanoscale. Doping these manganese oxides with other transition elements have
revealed as one of the most successful strategies to improve their properties
but a direct tool to determine where these dopants are located is still needed.
We present here our approach to the synthesis of doped ultrathin birnessite
nanoparticles and an atomic resolution electron microscopy study in order to
elucidate the positions that the foreign cations occupy.
Keywords: Birnessite,
Layered nanomaterials, HRTEM, Soft-chemistry, EELS
INTRODUCTION
Transition metal oxides form the
basis of a large number of functional devices that are part, among others, of
environmental, energy and information technologies. Many of these oxides
crystallize in structural types that can be modified by the introduction of
small compositional and/or structural variations. This aspect deserves
particular attention since ionic defects can play a key role in the development
or improvement of a certain functional behavior of these materials. On the
idea, well established, that slight variations in the composition can be the
origin of remarkable changes in a certain property, it should be noted that the
ease that have some structural types to promote the mobility of oxygen ions and
cause anionic deficiency, can lead to develop new strategies that could modify
or improve the functional behavior of many of these materials. Among the
transition metals, the best candidates to satisfy this behavior are those first
transition metals able to adopt several oxidation states and several coordination
environments. One of the most versatile is Mn, which due to the ability of
developing these capabilities, combined with its electronic behavior gives rise
to a wide range of applications as catalysts [1], ionic and electronic
conductors [2], magneto-resistant materials [3], etc. Most of these studies
have been devoted to three-dimensional manganese oxides, in particular those
related to the perovskite structural type. Nevertheless, in the last years
there has been an increasing activity in the search of more open structures
easing the interchange and ion mobility. In this scenario, manganese can also
be stabilised in layered structures. This is the case of birnessite related
compounds that can play a paramount role in catalytic and environmental
applications.
The birnessite-structured
manganese dioxide, δ-MnO2, is a polymorph of the MnO2
family. Its layered structure can be described as sheets of edge-sharing MnO6
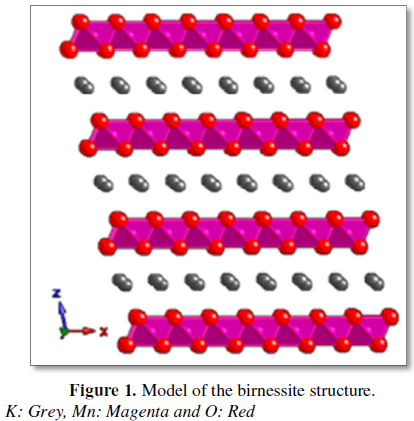
octahedra, interleaved by diverse cations and water molecules (Figure 1) [4]. The mineral that this
family is named after ((Na0.7Ca0.3)Mn7O172.8H2O)
usually has low crystallinity [5], so, the structural characterization of this
phase could not be possible until Post et al. [6] produced a synthetic sodium
birnessite. The δ-MnO2 crystallizes in a C2/m spatial group with the
following parameters: a=5.174 Å, b=2.850 Å, c=7.336 Å and β=103.18° [6]. The
oxidation state of manganese is III and IV, leading to charged layers. This
negative charge is compensated by interlaminar cations, usually alkali or
alkaline earth metals [6], but transition metal and rare earth interleaved birnessites have
also been
PROPERTIES AND APPLICATIONS
The natural birnessite
is an abundant phase in any kind of soils, especially in oceanic ones,
recovering deposits of other minerals as high specific surface aggregates [7].
The chemical influence of birnessite, like other manganese oxides, plays a key
role on regulation of heavy metals concentration in the waters of our planet.
These phases act as natural traps of metallic cations dissolved in rivers and
oceanic waters [11], thanks to their ability of absorbing them on their
structure. Therefore, the mineral birnessite is used in wastewater remediation
pools in mineral exploitations [12]. The high reactivity of these oxides also
benefits their environmental activity, mainly as oxidizing agent of different
pollutants. For example, birnessite is able to oxidize the toxic As(III) to
As(V), an specie much easier to eliminate by conventional water treatment
methods [13].
The most common
applications of the synthetic birnessite, like the natural one, make use of
their ionic exchange properties [14]. The birnessite phases, intercalated with
different monovalent cations, are able to reach topotactic exchange ratios of
80-90%. This means that the birnessite structure suffers only changes in its
basal distance between MnO2 layers, capturing and releasing a great
number of different kinds of dissolved cations. Therefore, nowadays, the main
technological application of synthetic birnessite is, again, heavy metals
removal from polluted waters.
The high ion mobility
in birnessite structure allows these phases to be used as electrodes for
lithium or sodium batteries [15,16]. In the former, depending on the synthesis
method, capacities in the order of 200 mA h•g-1 can be obtained
[17]. Nevertheless, these electrodes seem to suffer some degradation problems
but, after several charge/discharge cycles, the material keep capacities around
150 mA h•g-1 for long periods. The main efforts to improve the
electrochemical performance of these oxides are focused on the partial
substitution of Mn by other transition metals. For example, the inclusion of a
small amount of Ni in a lithium birnessite increases the electrode capacity to
213 mA h•g-1 even after 80 cycles [18]. Recently, Na ion batteries
have been intensively studied as an interesting alternative for certain
applications. In this field, birnessite based electrodes are again promising
candidates. The material response can be modulated by controlling the
intercalated water in the structure. Recent works, as the one published by Zhu
et al. [16], proof that highly hydrated sodium birnessites show a charge
capacity 190% greater than the dehydrated phase, keeping it after several
cycles.
Electrochemical capacitors, also known as supercapacitors, have shorter
charge/discharge times releasing much greater currents in shorter times and
larger operational life [19,20]. A sodium ion battery coupled with
supercapacitors could configure a flexible energy-storing device with multiple
applications. A supercapacitor is based on a double layer configuration that
stores energy in the solid electrolyte interlayer without chemical reactions.
Potassium birnessite shows a promising behavior since the bulk oxide, with very
low surface area, reach capacitance values of 110 F•g-1 [21]. The
performance of this material can be improved substituting some Mn for vanadium
[22]. Doping the oxide with 15% of V increases the capacitance to 246 F•g-1
after 200 cycles.
One of the most encouraging research fields on new energy conversion
systems are reversible solid-state fuel cells (RSOFCs). RSOFCs are single-unit,
all solid-state, electrochemical devices that can operate in both the fuel cell
(SOFC) and electrolysis (SOEC) mode. It is an energy conversion and storage
system especially appropriate to store intermittent renewable energy, such as
wind or solar [23]. A suitable candidate to integrate one of these devices have
to be active either in the oxygen evolution reaction (OER), when the cell is
operated in SOEC mode and oxygen reduction reaction (ORR) for the SOFC mode.
Birnessite oxides have been tested in both reactions separately [24,25].
Therefore, these compounds could constitute appropriate electrodes for RSOFCs
but, as far as we know, this possibility has not been explored. On one hand,
theoretical studies evidence that the interactions between the interlayer
cations and the water molecules diminish the OER activation energy. Therefore,
the electrocatalytic performance of the birnessite system can be improved
intercalating electrocatalytically active transition metals like Ni [8], Co
[26] or Cu [27]. On the other hand, like other MnO2 polymorphs,
birnessite oxides are active in ORR and have a significant electric
conductivity [25,28].
As it will be
discussed later, several different synthesis methods have been developed to
produce birnessite nanoparticles and, therefore, with high specific surface
area. This, combined with the known catalytic activity of other manganese
oxides, has encouraged the scientific community to test the catalytic behavior
of different birnessite phases on various reactions. The birnessite oxides are
active in formaldehyde oxidation. The molecule can be produced, under certain
humidity and temperature conditions, by degradation of wood furniture
varnishes. This can be avoided adding a small portion of birnessite oxide to
the preparation [9,29,30]. Birnessite phases are also active on CO oxidation,
improving their behavior when a portion of the manganese is substituted by
other transition metals [31]. Nevertheless, their low thermal stability limits
their activity.
SYNTHESIS METHODS AND REACTIVITY
There are numerous
synthesis methods to produce birnessite. The first procedure was based in a
solid-state reaction to obtain a bulk material [6] and, thereafter, there have
been some efforts to synthesize large monocrystals using flux methods [32].
Nevertheless, with the applications discussed above in mind, the birnessite
behavior could be improved with a nanostructured material. There are two soft
chemistry routes to obtain birnessite nanosheets. The first synthesis pathway
is a room-temperature co-precipitation method using manganese (II) sulfate or
nitrate in a basic media (usually a NaOH or KOH solution in order to produce
the sodium or potassium phase, respectively), in presence of an oxidizing agent
like hydrogen peroxide or sodium thiosulfate [33]. The second procedure
involves the reaction between potassium permanganate and hydrochloric acid at
80-100°C and its subsequent ageing at 50-60°C for 15 h [10]. The former yields
aggregated small nanoparticles, whereas the later leads to nanoflowers made by
birnessite nanosheets. Both methods allow the partial substitution of the
manganese by other elements. We have found in the literature Co [26], Cr [34]
and V [22] doped birnessite nanoparticles by these procedures, and our group,
as will be discussed later, have adapted the basic co-precipitation method to
produce Fe, Ti and Ce doped phases. While the co-precipitation method produces
the smallest nanoparticles and seems to be more flexible in terms of manganese
substitution, the KMnO4 reaction can be modified to synthesize complex
architectures. For example, Portehault et al. [10] designed core-corona
nanoparticles introducing Mn (II) in the acidic mixture and adjusting the aging
time and temperature. In this case, the particles are made by an amorphous MnO2
core surrounded by birnessite nanosheets. Zhu et al. [35] produce ultrathin
parallel birnessite nanosheets grown onto MnO2 nanowires; aging the
acidic KMnO4 solution with MnOH nanowires as sheds.
Apart from these two
common methods, some other synthesis pathways have been developed. Ching et al.
[36] published a sol-gel method using KMnO4 and glucose to form the
gel that is subsequently calcined. Nevertheless, this procedure yields larger
particles than the previous ones. There are also hydrothermal routes studied
for this system, like the one proposed by Zhang et al. [37]. This method drives
to porous birnessite nanoflowers with high specific surface area for their
application as supercapacitors. Komaba et al. [38] found that, while testing
the electrochemical performance of some Mn (III) based oxides, the activity of
the material was significantly improved after the first cycle. The structural
analysis revealed that the initial material had been modified producing birnessite
nanoparticles, being this new phase responsible of the improved behavior.
Like other 2D
materials, birnessite can undergo ion exchange reactions and, under certain
conditions, the structure can be delaminated into disperse monolayers. The ion
exchange reactions take place simply by soaking the oxide with a concentrated
solution of the cation that wants to be intercalated. In this way, Co [24], Ni
[8] or Cu [27] interleaved birnessites can be prepared. When the intercalated
cation is large enough, mostly bulky amines like tetrabutylammonium (TBA), the
interaction between layers is weak enough that the monolayers can be suspended
in the appropriate solvent [39,40].
Other interesting
feature of the birnessite related oxides is that can be transformed into different
channel MnO2 structures by a hydrothermal method [33]. The final
channel structure depends on which cation occupies the interlayer space, the
temperature and the acid used in the hydrothermal method. Although the exact
mechanism is still unknown, the layer structure seems to collapse around the
interlayer cation forming the channel along the final structure. The size of
this channel is templated by the interlayer cation. So, starting from a
compositionally controlled birnessite, a well-defined tunnel structured
manganese oxide can be achieved. These tunnel structures, like pyrolusite or
hollandite, are well known as promising functional materials [41,42] and their
particle size, morphology and composition could be controlled by using the
right birnessite precursor.
SEARCHING FOR THE DOPANT LOCATION
Our current work is
focused on either synthesize nanostructured birnessites doped with Fe, Ti and
Ce and shed light over where the dopants are located by using atomic resolution
electron microscopy. The obtained birnessites are meant to be used as solid
precursors for hollandite structured nanowires. As previously mentioned, these
tunnel structures have attractive properties, their catalytic activity being
one of the most relevant [42]. Besides, as previously mentioned, Co, Cr and Cu
doped birnessite phases have also been reported. On the basis of this
information, we thought that Fe or Ti, known catalytically active elements
[43,44], could be adequate dopants to improve the performance of the final
material. Ce is also a quite catalytically active element, and its inclusion in
the manganese oxide framework can enhance its behavior. At the same time, we
find that, as far as we know, there is some lack of precision defining where
the dopants are located in the oxide structure. Most authors rely on indirect
methods to induce where the dopants are but, even when they are probably right;
their conclusions are subject to interpretation. We have tried to find the
appropriate conditions for a direct visualization of the structure of the
birnessite nanoparticles by atomically resolved transmission electron
microscopy (HRTEM) and, at the same time, analyze their local composition by
scanning transmission electron microscopy (STEM) and electron energy loss
spectroscopy (EELS).
We have chosen the
co-precipitation method to produce our birnessite nanoparticles. The procedure
consists in the injection of a 1 M Mn(NO3)2•4H2O
(99.99%, Sigma-Aldrich) solution into a mixture of 50 ml KOH (99.99%,
Sigma-Aldrich) solution 0.8 M with 1.02 ml of H2O2 (30%,
Sigma-Aldrich) at room temperature under vigorous stirring. A brownish black
precipitate forms immediately, which is washed with water and recovered by
centrifugation. In order to produce Fe, Ti or Ce doped phases, the manganese
nitrate solution should be substituted by the corresponding mixture of this
precursor plus Fe(NO3)3•9H2O (99.99%,
Sigma-Aldrich), dihydroxybis(ammoniumlactate)titanium (IV) (sol. 50% in water,
Sigma-Aldrich) or Ce(NO3)3 (99.99%, Sigma-Aldrich),
respectively.
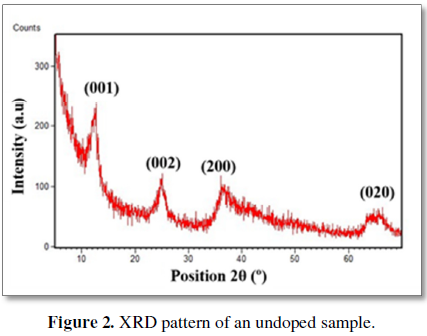
The average composition of the undoped phase, determined by electron microscopy probe and thermogravimetric analysis, is K0.36MnO2•0.87H2O. X-ray diffraction (XRD) data show low intensity wide diffraction maxima (Figure 2) which can be indexed as a birnessite phase (COD 9001272). In the case of the doped phases, the compositional analysis is in agreement with the nominal one. When Fe, Ti or Ce is included in the reaction mixture, the X-ray diffraction pattern shows even wider maxima (not included). The wide nature of the diffraction maxima suggests the presence of nanostructured phases.
TEM study, performed
in two JEOL electron microscopes, JEM 2100 and GRAND ARM, confirms the
stabilization of doped and undoped birnessite nanoparticles. Typical low
magnification TEM images indicate the presence of very thin and heterogeneous
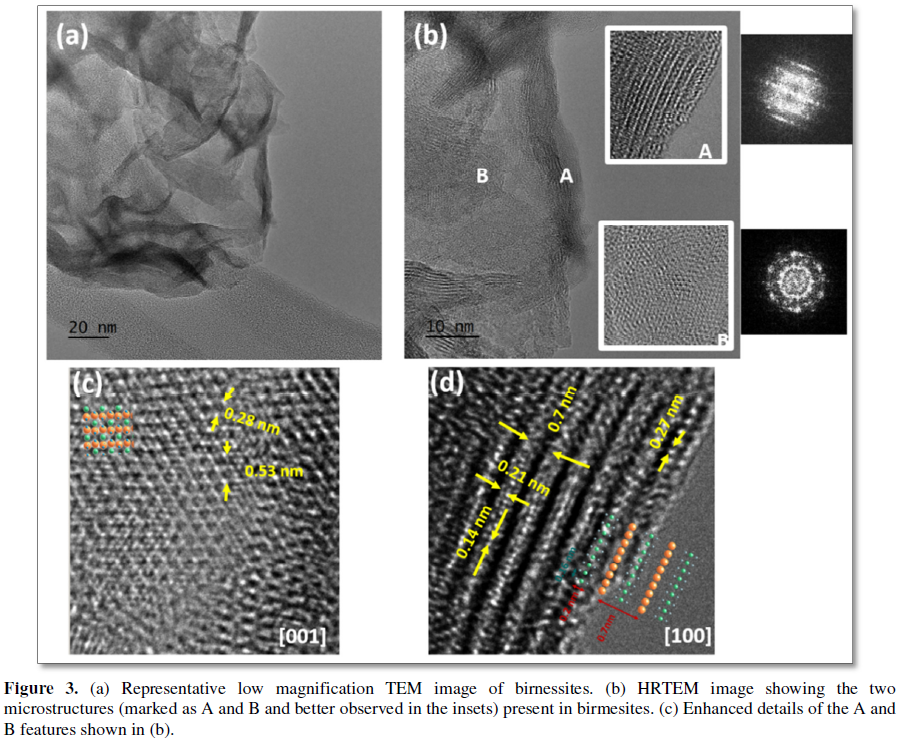
shaped aggregates (Figure 3a) which,
according to HTREM images, comprise two microstructural features (marked in Figure 3b as A and B together with
enhanced inset and their corresponding FFT):
A) A polycrystalline
matrix, built up from very small particles (5 nm or less), exhibiting a
hexagonal distribution of contrasts with 0.53 nm ´
0.28 nm periodicities, in agreement with the birnessite unit cell along (001) (Figure 3c).
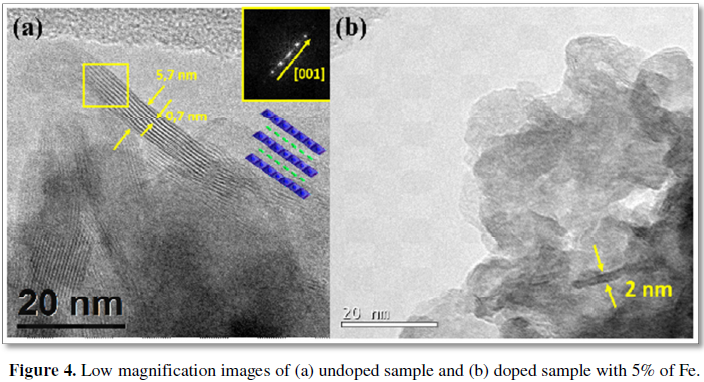
B) Layered nanostructures, built up from 2-5 layers with typical interlayer distances around 0.7 nm, characteristic of the birnessite lattice along the (100) direction (Figure 3d). The microstructural features here shown correspond to an iron doped sample (5% Fe) but they are present in both doped and undoped samples. Nevertheless, it should be mentioned that, as the dopant is introduced, the ratio of particles orientated perpendicular to the (001) direction increases, which is in agreement with the broadening of the diffraction peaks observed in XRD (Figure 4). This could be explained due to a reduction of the particle size and aspect ratio as a consequence of the inclusion of the dopants, as previously observed in other layered oxides [45].
In order to get further structural and compositional information about
the cation distribution as well as the transition metal oxidation state, an
atomically resolved study was performed in an aberrated corrected Scanning
Transmission Electron Microscope (STEM) JEOL JSM-ARM200cF microscope (Cold
Emission Gun) combining the HAADF (high annular angle dark field) imaging
technique and EELS spectroscopy (Quantum Gif spectrometer). HAADF implies the
collection of the electrons scattered at high angles (Solid semi-angles between
68-280 mRad) resulting in incoherent imaging. Under these experimental
conditions, the scattered intensity is approximately proportional to Z1.83
(Z=atomic number of the elements in the sample). In this sense, the intensity
differences among columns must be related to the different cationic composition,
providing images where the column contrast can be qualitatively interpreted as
chemical information: the brighter contrast would correspond to heaviest
elements while less bright contrast to those elements with lower Z. The
incorporation of spherical aberration correctors allows acquiring HAADF images
with atomic resolution, making possible to resolve and locate cationic columns
with different atomic number, being then an ideal technique for determining the
dopant location in original birnessite. Nevertheless, it should be mentioned
the high instability of the samples under the focused electron beam, especially
in the doped samples, making difficult their characterization. In this
scenario, we have explored the use of different acceleration voltages in the
range of 200 to 80 kV, being the best results obtained setting the voltage to
80 kV and low electron doses.
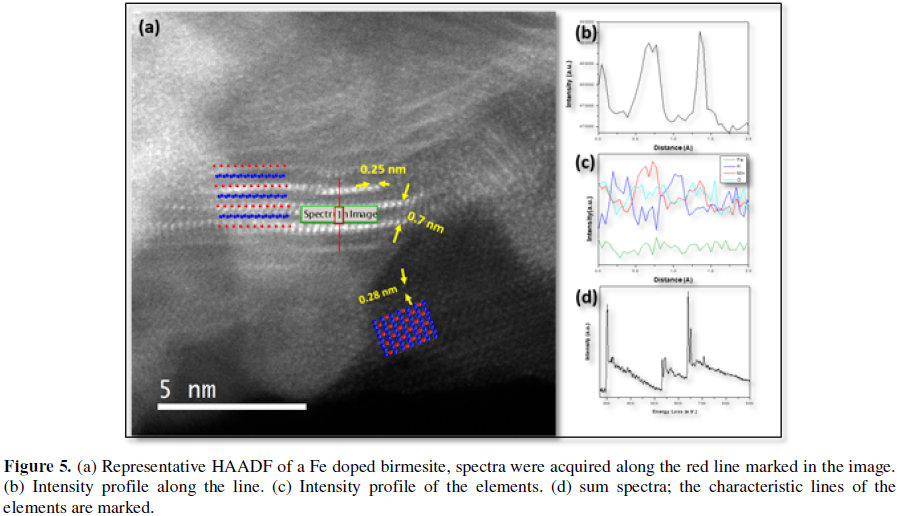
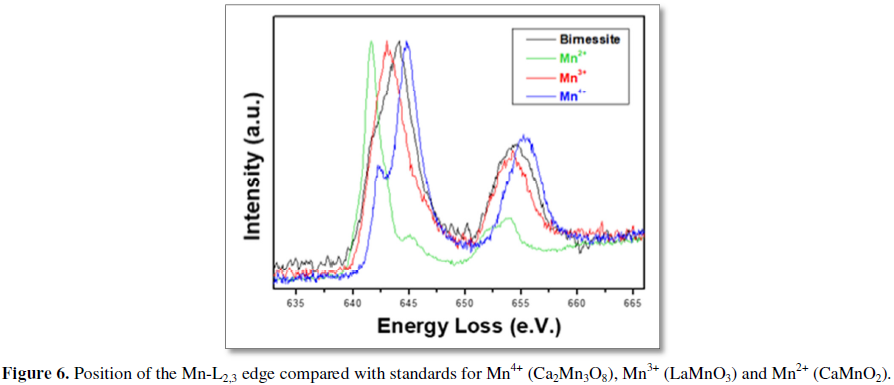
A typical HAADF image of an iron doped birnessite (Figure 5a) reflects, again, the presence of a very thin matrix oriented along (001) direction and small pieces of layered nanoflakes comprising, in this case, no more than two layers, along the perpendicular direction (schematic structural models have been included). According to the above description of Z contrast imaging, the brightest contrasts must correspond to the heaviest cations, i.e., Mn (Z=25) and/or Fe (Z=26), whereas K (Z=19), in the interlayer space, is not observed due to its low concentration as well as the near presence of Mn/Fe, in the adjacent layers. Nevertheless, it is clear that, in this interlayer position there are not bright contrasts indicating the presence of dopant, i.e., Fe. These experimental observations suggest that Fe should be substituting Mn. The next logical step is trying to elucidate the atomic distribution of the elements simultaneously acquiring the EELS spectra on the area of the layers shown in HAADF. Nevertheless, this is not possible because of the severe damage that this kind of small areas suffers while the beam is scanned during the required time to obtain a good signal-noise ratio spectrum-imaging. Even using short acquisition times (less than 1 min), the scanned area is always destroyed. However, if the EELS spectra are acquired along a line instead of an area, during total exposition times around 30 s, the nanoflakes are not severely damaged. The corresponding EELS sum spectra (Figures 5b-5d), simultaneously acquired along the line marked in the image, evidence the characteristic adsorption edge of the dopant cation, Fe (L3-708 eV), in addition to those of K (L3-296 eV), O (K-510 eV) and Mn (L3-640 eV) of the starting birnessite compound. In addition, the L2,3 Mn fine structure has been analyzed in order to get information of the Mn oxidation state. For that purpose, the position (Figure 6) and L2,3 intensity ratio (Table 1) of the Mn-L2,3 edge were compared with standards for Mn4+ (Ca2Mn3O8), Mn3+ (LaMnO3) and Mn2+ (CaMnO2). Both methods, suggest the coexistence of Mn3+ and Mn4+, as can be seen in Figure 3 and Table 1, since the Mn L2,3 edge position and L2,3 intensity ratio of the sample is in between the standards for Mn3+ and Mn4+. This result has been obtained either for doped or undoped samples. The oxidation state of Fe cannot be evaluated due to the low signal to noise ratio as consequence of the low Fe concentration.
CONCLUSION
The ensemble of these
results shows the optimization of the synthesis and characterization conditions
to obtain reproducible nanostructured birnessite doped manganese oxides and to
elucidate where these dopants are hosted. Actually, ultrathin (2-4 layers) 5 nm
birnessite nanoplatelets, containing Fe, Ti and Ce, can be directly obtained by
a room-temperature basic co-precipitation method. The microstructural
characterization by means of TEM indicates that the introduction of the dopants
previously mentioned leads to the decrease of the particle size of the pure Mn
birnessite promoting the orientation of the structure along the c axis. Local
EELS compositional analysis confirms the incorporation of dopants in the
layered birnessite structure, as well as the coexistence of Mn3+ and
Mn4+ oxidation states. Although it has not been possible to acquire
atomically resolved images and EELS chemical maps due to beam damage, the HAADF
images of birnessite nanoplatelets oriented along (100) suggest that the
interlayer space is free of transition metal atoms. Therefore, the dopants
should be located in the manganese positions.
These characterization
techniques and synthesis methods can provide an effective tool to design robust
functional materials based on birnessite manganese oxide. In the particular
case of the electrochemical behavior, the use of these state of art tools could
lead to further enhancement of its performance placing these materials as one
of the most promising and economic candidates for the next generation of
RSOFCs.
ACKNOWLEDGEMENT
This work was
supported by the Spanish Ministry of Innovation, Science and Technology and
Spanish Ministry of Economy and Competitiveness through Research Projects
MAT2014-52405-C02-01 and MAT2017-82252-R.
1.
Royer S, Duprez D, Can F, Courtois X, Batiot-Dupeyrat
C, et al. (2014) Perovskites as substitutes of noble metals for heterogeneous
catalysis: Dream or reality. Chem Rev 114: 10292-10368.
2.
Sengodan S, Choi S, Jun A, Shin TH, Ju YW, et al.
(2014) Layered oxygen-deficient double perovskite as an efficient and stable
anode for direct hydrocarbon solid oxide fuel cells. Nat Mater 14: 205-209.
3.
Cortés-Gil R, Ruiz-González ML, Alonso JM,
García-Hernández M, Hernando A, et al. (2011) Magnetoresistance in La0.5Sr0.5MnO2.5.
Chem Eur J 17: 2709-2715.
4.
Yang X, Tang W, Feng Q, Ooi K (2003) Single crystal
growth of birnessite- and hollandite-type manganese oxides by a flux method.
Cryst Growth Des 3: 409-415.
5.
Kingsbury AWG (1956) The rediscovery of Churchite in
Cornwall. Mineralogical Magazine 31: 282-282.
6.
Post JE, Veblen DR (1990) Crystal structure
determinations of synthetic sodium, magnesium and potassium birnessite using
TEM and the Rietveld method. Am Mineral 75: 477-489.
7.
Post JE (1999) Manganese oxide minerals: Crystal
structures and economic and environmental significance. Proc Natl Acad Sci U S
A 96: 3447-3454.
8.
Thenuwara AC, Cerkez EB, Shumlas SL, Attanayake NH,
McKendry IG, et al. (2016) Nickel confined in the interlayer region of
birnessite: An active electrocatalyst for water oxidation. Angew Chem Int Ed
55: 10381-10385.
9.
Zhu L, Wang J, Rong S, Wang H, Zhang P (2017) Cerium
modified birnessite-type MnO2 for gaseous formaldehyde oxidation at
low temperature. Appl Catal B 211: 212-221.
10.
Portehault D, Cassaignon S, Baudrin E, Jolivet JP
(2008) Design of hierarchical core-corona architectures of layered manganese
oxides by aqueous precipitation. Chem Mater 20: 6140-6147.
11.
Jenne EA (1968) Trace inorganics in water. USA:
American Chemical Society.
12.
Prasad VS, Chaudhuri M (1995) Removal of bacteria and
turbidity from water by chemically treated manganese and iron ores. Aqua 44:
80-82.
13.
Driehaus W, Seith R, Jekel M (1995) Oxidation of
arsenate (III) with manganese oxides in water treatment. Water Res 29: 297-305.
14.
Prieto O, del Arco M, Rives V (2003) Characterization
of K, Na and Li birnessites prepared by oxidation with H2O2
in a basic medium. Ion exchange properties and study of the calcined products.
J Mater Sci 38: 2815-2824.
15.
Bach S, Pereira-Ramos JP, Baffier N (1995) Synthesis
and characterization of lamellar MnO2 obtained from thermal
decomposition of NaMnO4 for rechargeable lithium cells. J Solid State Chem 120:
70-73.
16.
Zhu K, Guo S, Li Q, Wei Y, Chen G, et al. (2017)
Tunable electrochemistry via controlling lattice water in layered oxides of
sodium-ion batteries. ACS Appl Mater Interfaces 9: 34909-34914.
17.
Yagi H, Ichikawa T, Hirano A, Imanishi N, Ogawa S, et
al. (2002) Electrode characteristics of manganese oxides prepared by reduction
method. Solid State Ion 154-155: 273-278.
18.
Wang H, Li X, Zhou Q, Ming H, Adkins J, et al. (2014)
Diversified Li1.2Ni0.2Mn0.6O2
nanoparticles from birnessite towards application specificity and enhancement
in lithium-ion batteries. J Alloys Compd 604: 217-225.
19.
Conway BE, Pell WG (2003) Double-layer and
pseudocapacitance types of electrochemical capacitors and their applications to
the development of hybrid devices. J Solid State Electrochem 7: 637-644
20.
Wang YG, Wang ZD, Xia YY (2005) An asymmetric supercapacitor
using RuO2/TiO2 nanotube composite and activated carbon
electrodes. Electrochim Acta 50: 5641-5646.
21.
Brousse T, Toupin M, Dugas R, Athouël L, Crosnier O,
et al. (2006) Crystalline MnO2 as possible alternative to amorphous
compounds in electrochemical supercapacitors. J Electrochem Soc 153:
A2171-A2180.
22.
Liu L, Min M, Liu F, Yin H, Zhang Y, et al. (2015)
Influence of vanadium doping on the supercapacitance performance of hexagonal
birnessite. J Power Sources 277: 26-35.
23.
Laguna-Bercero MA (2012) Recent advances in high
temperature electrolysis using solid oxide fuel cells: A review. J Power
Sources 203: 4-16.
24.
Thenuwara AC, Shumlas SL, Attanayake NH, Aulin YV,
McKendry IG, et al. (2016) Intercalation of cobalt into the interlayer of
birnessite improves oxygen evolution catalysis. ACS Catal 6: 7739-7743.
25.
Meng Y, Song W, Huang H, Ren Z, Chen SY, et al. (2018)
Structure-property relationship of bifunctional MnO2 nanostructures:
Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction
reaction catalysts identified in alkaline media. J Am Chem Soc 136:
11452-11464.
26.
McKendry IG, Thenuwara AC, Shumlas SL, Peng H, Aulin
YV, et al. (2018) Systematic doping of cobalt into layered manganese oxide
sheets substantially enhances water oxidation catalysis. Inorg Chem 57:
557-564.
27.
Thenuwara AC, Shumlas SL, Attanayake NH, Cerkez EB,
McKendry IG, et al. (2015) Copper-intercalated birnessite as a water oxidation
catalyst. Langmuir 31: 12807-12813.
28.
Huang H, Meng Y, Labonte A, Dobley A, Suib SL (2013)
Large-scale synthesis of silver manganese oxide nanofibers and their oxygen
reduction properties. J Phys Chem C 117: 25352-25359.
29.
Wang J, Li J, Jiang C, Zhou P, Zhang P, et al. (2017)
The effect of manganese vacancy in birnessite-type MnO2 on room-temperature
oxidation of formaldehyde in air. Appl Catal B 204: 147-155.
30.
Selvakumar S, Nuns N, Trentesaux M, Batra VS, Giraudon
JM, et al. (2018) Reaction of formaldehyde over birnessite catalyst: A combined
XPS and ToF-SIMS study. Appl Catal B 223: 192-200.
31.
Shen YF, Suib SL, O’Young CL (1996) Cu containing
octahedral molecular sieves and octahedral layered materials. J Catal 161:
115-122.
32.
Yang X, Tang W, Feng Q, Ooi K (2003) Single crystal
growth of birnessite- and hollandite-type manganese oxides by a flux method.
Cryst Growth Des 3: 409-413.
33.
Feng Q, Yanagisawa K, Yamasaki N (1998) Hydrothermal
soft chemical process for synthesis of manganese oxides with tunnel structures.
J Porous Mater 5: 153-161.
34.
Ching S, Driscoll PF, Kieltyka KS, Marvel MR, Suib SL (2001)
Synthesis of a new hollandite-type manganese oxide with framework and
interstitial Cr(III) Chem Commun 23: 2486-2487.
35.
Zhu S, Li L, Liu J, Wang H, Wang T, et al. (2018)
Structural directed growth of ultrathin parallel birnessite on β‑MnO2
for high-performance asymmetric supercapacitors. ACS Nano 12: 1033-1042.
36.
Ching S, Petrovay DJ, Jorgensen ML (1997) Sol-gel
synthesis of layered birnessite-type manganese oxides. Inorg Chem 36: 883-890.
37.
Zhang X, Miao W, Li C, Sun X, Wang K, et al. (2015)
Microwave-assisted rapid synthesis of birnessite-type MnO2
nanoparticles for high performance supercapacitor applications. Mater Res Bull
71: 111-115.
38.
Komaba S, Tsuchikawa T, Ogata A, Yabuuchi N, Nakagawa
D, et al. (2012) Nano-structured birnessite prepared by electrochemical
activation of manganese(III)-based oxides for aqueous supercapacitors.
Electrochim Acta 59: 455-463.
39.
Liu Z, Ooi K, Kanoh H, Tang W, Tomida T (2000)
Swelling and delamination behaviors of birnessite-type manganese oxide by
intercalation of tetraalkylammonium ions. Langmuir 16: 4154-4164.
40.
Liu Z, Ma R, Ebina Y, Takada K, Sasaki T (2007)
Synthesis and delamination of layered manganese oxide nanobelts. Chem Mater 19:
6504-6512.
41.
Perreault P, Rifflart S, Nguyen E, Patience GS (2016)
Pyrolusite: An alternative oxygen carrier for chemical looping combustión. Fuel
185: 630-638.
42.
Pahalagedara L, Kriz DA, Wasalathanthri N, Weerakkody
C, Meng Y, et al. (2017) Benchmarking of manganese oxide materials with CO
oxidation as catalysts for low temperature selective oxidation. Appl Catal B
204: 411-420.
43.
Theofanidis SA, Galvita VV, Konstantopoulos C, Poelman
H, Marin GB (2018) Fe-based nano-materials in catalysis. Materials 11: 831.
44.
Davis-Gilbert ZW, Tonks IA (2017) Titanium redox
catalysis: Insights and applications of an earth-abundant base metal. Dalton
Trans 46: 11522-11528.
45.
Azor A, Ruiz-Gonzalez ML, Gonell F, Laberty-Robert C,
Parras M, et al. (2018) Nickel-doped sodium cobaltite 2D nanomaterials:
Synthesis and electrocatalytic properties. Chem Mater 30: 4986-4994.