905
Views & Citations10
Likes & Shares
With
pharmaceutical companies’ repeated failures at finding effective interventions
for Alzheimer’s disease, together with an increasing reliance on the growing
Federal funding for research, there is an emergent opportunity for financing
alternate research through crowdfunding. Crowdfunding—where funding is obtained
from small donations from a large group of people—has become a new source of
funding for medical research. By understanding how the research community has
evolved to study Alzheimer’s disease the pitfalls of this strategy can be
highlighted. Alzheimer’s disease research is complex. From its inception in the
early 1900s, Alzheimer’s disease has been at the center of movement within
psychiatry to define the disease on the basis of its biology. Recent
emphasis—through the DSM (Diagnostic and Statistical Manual of Mental
Disorders), RDoC (Research Diagnostic Criteria), RDoC (Research Domain
Criteria) as well as the more recent Framework from the U.S. National Institute
on Aging—have supported an exclusive emphasis on biology. But by excluding
other aspects of the disease, such as its clinical expression, this research
approach will be shown to be faulty and contradictory. So far this approach has
resulted in 100% failures. By examining the historical and financial
circumstances of the industry centered on Alzheimer’s disease a strong warning
is given to the public to mistrust crowdfunding Alzheimer’s disease research. A
broader and more inclusive approach is likely to generate a better
understanding of the disease and therefore hold better promise for
understanding the disease in the long term. Such a nuance approach competes
badly with the more binary search for a cure and is less receptive to public
support through crowdfunding.
Keywords: Crowdfunding, Alzheimer’s disease, Medical research
INTRODUCTION
The scientific method is based on two
precepts. It must summarize past research by consolidating this body of
knowledge into a theory, and it must be able to generate hypothesis (questions
or predictions) from this theory that can be tested and which can be refuted.
Observations within this scientific method ultimately improves theory and forms
the primary distinction between science and metaphysics, myths or tautological.
Popper [1] in his book Conjectures
and Refutations argued that by their
function scientific theories must upset accepted views of the world. Scientists
are necessarily radicals. They must work to overthrow accepted doctrines as
part of their scientific purpose. If we know a phenomenon completely then
science no longer has a function. Science is a method for acquiring knowledge
(epistemology) that is accomplished through the development and then
falsification of theories. Which is why we have an alternate hypothesis in
scientific experiments since we can disprove a scientific theory (by accepting
the null hypothesis) but we can never prove it (cannot accept the alternate
hypothesis). Science, according to Popper [1], evolves by observations
eliminating weak theories by proving them as false.
More than half a century ago, at the same
time that Popper [1] was writing about these percepts of scientific progress in
the 1960s, Kuhn [2] was writing about how science was being conducted and
managed. In his 1962 book The Structure
of Scientific Revolutions, Kuhn [2] determined that the reason for the erratic
progress of science was because of social factors. Kuhn [2] describes how even
when hypothesis are falsified, there is enough invested interest in maintaining
the given theory (i.e., the status quo) that this proof of falsification is
ignored at best and disparaged at worse. Only when there is un-refutable and
overwhelming evidence that a revolution takes place to overthrow the older
theory in favor for the new one. The process of scientific progress mirrors
This paper attempts to understand the progress
of Alzheimer’s disease research over the last 70 years using these two metrics
of scientific progress. The aim is to chart an alternate path for research and
to understanding the social aspects of conducting research in Alzheimer’s
disease. The insights afforded by evaluating Alzheimer’s disease research
through these prisms will provide clearer understanding of the type of barriers
that are still holding back the science. By identifying any barriers a clearer
path might be exposed that should accelerate progress to understanding the
disease. Alzheimer’s disease research is at the breaking point of overthrowing
the old theory and replacing it with a new broader theory. However continuing
funding for the old theory with the possible inclusion of crowdfunding will
delay and impede this necessary transition.
Crowdfunding through sites such as Gofundme,
Kickstarter, Indiegogo, Fundly, JustGiving, Rockethub and Facebook all have
fundraisers for some aspects of Alzheimer’s disease activity. Some even focus
on research and promote trials on potential cures such as Petridish, #SciFund
and Experiment.com (renamed from Microryza). Experiment.com is currently the
largest dedicated platform for crowdfunding research [3]. In a 2018 review of
crowdfunding in research, Sauermann et al. [3] reported that most of the
activities involved scientific investigation (78%) and were mainly concentrated
in the U.S. (89%) and the majority (80%) affiliated with universities and
colleges. This is not surprising since U.S. universities are adept at
fundraising campaigns. Most of these research crowdfunding events were in the
fields of social sciences and psychology and tended to promote undergraduate or
master’s students (30%) followed by PhD or MD students (25%). Overall through
one website alone Experiment.com projects raised a total of $4.37 million, with
the average project raising $6,425. Such numbers are miniscule compared to the
$2.3 billion budget of the U.S. National Institute on Aging but it is a trend
that shows incredible growth. Especially since crowdfunding is attracting
junior faculty/researchers as their success rate for crowdfunding is higher
than traditional sources of funding.
Crowdfunding complements other public
participation in science especially “crowd science” or “citizen science”
projects. These projects increase the permeability between scientists and the
public who contribute their time (e.g. collecting samples or observing events),
resources (e.g. computer power) and knowledge (e.g. experiences and feedback).
But such participation is prescribed and relies on binary tasks that do not
require complex chores or decisions. With a complex scientific problem,
enticing public support would require making the problem seem far simpler than
it is. Alzheimer’s disease is now at that stage of simplification. Any federal
source of information on Alzheimer’s disease mimics the same interpretation as
the 2018 framework which culminates a century of assumptions about the disease:
that two misfolded proteins cause the disease [4]. There remains great
resistance from the status quo—a cabal of prominent researchers and
administrators that have built their careers and business on this one specific
hypothesis related to Alzheimer’s disease—to change the dominant theory in
research. Understanding this dominance provides an insight into how to untangle
the political and the business from the science in Alzheimer’s disease
research.
THE PROBLEM
Alzheimer’s disease is one type of
dementia—an umbrella term that encompasses many types of specific brain atrophy
diseases—that also include the less common vascular dementia, Lewy bodies and
Fronto-temporal dementia as well as other neurological brain diseases. There
are other “comorbid neurological diseases” that affect the brain, more prevalent
than Alzheimer’s disease and had these conditions been known before their death
“would likely have affected their treatment before death” [5; p.35]. Dementia
is too broad and too quick a diagnosis, but it was not always like this.
Alzheimer’s disease was baptized in 1910 as a
disease by Emil Kraepelin—Alois Alzheimer’s supervisor—who included
“Alzheimer’s disease” as a new unique disease in the eighth edition of his book
Psychiatre. Alois Alzheimer linked amyloid beta deposition and pathologic tau
with dementia in a 45 year old Auguste Deter who died six years later. While
Alzheimer’s disease continues attracting greater and greater interest there is
a warning in this attraction of focusing on one disease. Auguste Deter died
from infections from bedsores a most painful death and one that is preventable
[6]. To this day we continue focusing on the disease while ignoring the
patient.
Although there are many potential alternate
approaches to developing research guidelines in Alzheimer’s disease in 2018 the
NIA relapsed back to a much narrow definition of the disease [7-12]. This new
Research Framework: Toward a biological definition of Alzheimer’s disease
headed by Jack et al. [4] (referred to as the Framework) embraces a piecemeal
framework that focuses on two biological markers correlated with Alzheimer’s
disease while discounting the clinical expression of the disease. For the first
time the clinical aspect of the disease—what we think of as Alzheimer’s
disease—how it is expressed through memory loss, changes in mental capacities
and mood and personality changes—will be ignored. In contrast to the earlier
2011 guidelines [13], the new Research Framework favors three types of
information: [A] amyloid beta deposition, [T] pathologic tau and [N]
neurodegeneration. This new AT(N) definition exclusively relies on the presence
of biological markers to define the disease. It is a tautological argument,
Alzheimer’s disease is defined by its biology and the biology defines the
disease. There is no way to refute this theory. Such a model, promoted by a
U.S. Federal scientific agency, cannot be tested. Popper would argue that such
arguments are not science but rather metaphysical. Exploring the reasons for
promoting such pseudo-science leads to conflicts of interests among the primary
authors of this new Framework. But a more insidious and pervasive argument is
more nuanced and involves a historical predisposition to focus on biological
determinism within psychiatry. Both these reasons highlight what Kuhn would
call “development-by-accumulation” not for scientific but for political and
economic purposes. Scientists are weakening the scientific process for
political and/or economic gain.
CONFLICTS OF
INTEREST
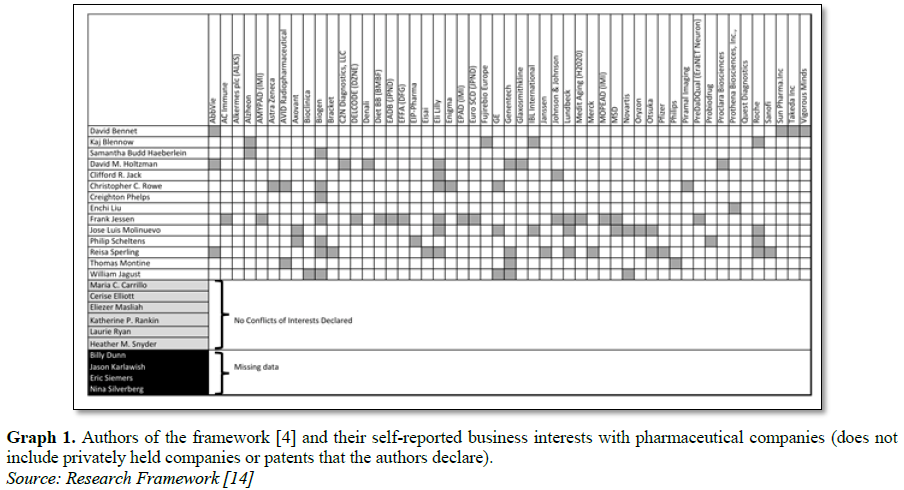
In a supplemental attachment to the framework
[14], a list of conflicts of interest activities can be indexed. From this list
(Graph 1) we can see three main
results.
Out of 24 authors, only six report no
conflicts of interests (25%) while four had no data or missing information from
the source document (17%). For the majority 14 authors of the paper (58%) had
multiple recent connections with pharmaceutical industry that benefit from Alzheimer’s
disease. These 14 authors reported 79 separate business or economic benefits
with pharmaceutical companies (average of 5.6 per author.) In addition, three
authors hold current patents that directly benefit from the approach being
promoted by their manuscript. In contrast, in 2001 the highest French
administrative court (Conseil d’Etat) requested the immediate withdrawal of
guidelines on dementia elaborated by the French National Health Authority
(Haute Autorité de Santé) owing to undisclosed serious conflict of interest for
panel members [15]. The argument is if you disclose conflicts of interests does
this disclosure diminish the conflict and reduce the interest in competing
business?
The authors have argued that these federal declarations are Guidelines [13] or Frameworks [4] and therefore hold no binding influence. But this attitude conflicts with the reality of research. Because the Framework is published under the NIA auspices it forms the basis for NIA funding in Alzheimer’s disease research. The majority of funding is allocated to studies that are within the dictates of these de facto theories. In reality, these are pseudo-science as they fund research that look for confirmation rather than refutation. Popper is more flippant when he writes “It is easy to obtain confirmations or verifications, for nearly every theory—if we look for confirmations” [1, p.3]. The foundation for such hubris goes much deeper. Especially with Alzheimer’s disease there is a particular penchant to associate the disease purely with biological correlates. From its inception Alzheimer’s disease was an important disease because it made such bold biological assertions from the start. The disease affects older people and has traditionally remained on the periphery of avant garde research. Alois Alzheimer’s specialty was in fact syphilis, a bacterial infection that resulted in a terminal stage of neurosyphilis, a type of dementia. The attraction of Alzheimer’s disease was that the same biological assertion could be made.
BIOLOGICAL
DETERMINISM
Such scientific arrogance has been evolving
for a century. At the turn of the 1900s academic disciplines were separating
into distinct areas of study. In mental sciences, Emil Kraepelin, together with
Eugen Bleuler, developed a more biological path for the nascent discipline of
psychiatry through their work with schizophrenia and later Alzheimer’s disease.
This occurred at a time when much stronger forces—primarily the psychoanalysts
championed by Sigmund Freund, and experimental psychologists championed by
Wilhelm Wundt—were succeeding in redefining mental health as unresolved
psychological trauma. Psychiatry was left with explaining mental illness as a
chemical/biological imbalance. But at the time very little was known about such
biological processes and as a result psychiatry was relegated to classifying
diseases.
The 1880 U.S. Census only distinguished seven
categories of mental illness: mania, melancholia, monomania, paresis, dementia,
dipsomania, and epilepsy. Within this tangle of disorders, Kraeplein
differentiated between premature (praecox) dementia (which we now called
schizophrenia) and ‘manic depression’ as two separate forms of psychosis.
Kraepelin was not the first to make such a distinction but he was the first to
argue that schizophrenia is a biological illness caused by anatomical or toxic
processes (as yet unknown.) Although Arnold Pick in 1891 defined schizophrenia
as a psychotic disorder (hebephrenia) in 1911, Eugen Bleuler revised this idea,
renaming ‘dementia praecox’ (premature dementia) as schizophrenia.[15] Together
Kraepelin and Bleuler created a new emphasis of biological psychiatry—an
emphasis that remains today. It marked a paradigm change in psychiatry, from a
classification of diseases based on "symptoms" to one based on
(assumed) neurological causes.
Throughout the history of nosology—the branch
of science dealing with the classification of disease—the aim has been to
define a more reliable and valid diagnosis. But the process was not linear as
many diagnoses proved difficult. Our present nosology has been significantly
influenced by the Diagnostic and Statistical Manual of Mental Disorders, or
known by its acronym DSM. Most versions of the DSM aim at improving both the
reliability and validity of categorizing specific disease to help with
diagnosis. Other international classification systems exist including one
coordinated by the United Nations, World Health Organization as the
International Classification of Diseases (ICD). The DSM is not restricted to
some clinical tool for diagnosticians. Emerging as the ultimate clinical
reference manual the DSM also forms the foundation for residency training; it
is used to define reimbursement by insurance companies; it is used to evaluate
eligibility to accessing social and medical services; and it forms the basis
for defining criminal culpability in courts of law [17]. The DSM is a veritable
tool that defines significant aspect of our medical interaction.
First introduced in 1952, the DSM-I proved to
be limited, ill applied and too broad. Although each subsequent version
represented incremental improvements—up to the latest version V introduced in
2013 comprising 541 different diagnoses—the most radical change happened in
1980 with the DSM-III. The DSM-III established a more biological approach to
diagnoses, elevating psychiatric disorders to neurological diseases and moved
the focus of therapy from psychotherapy to medication [18,19].
The reverberations from such change in
emphasis are still felt today with the push to recognize schizophrenia as a
neurological disorder—involving damage to and degeneration of the nervous
system—rather than a psychiatric one [20]. Eventually the classification of
both DSM-II and the ICD-8 became synchronized making a powerful testament of
solidarity. However there was pushback. In particular two studies exposed their
lack of reliability and validity. A 1971 paper comparing U.S. with British
diagnostic practices reported a general carelessness among U.S. diagnostician
in their application of the DSM-II [21]. This was followed by a study by
Rosenhan in 1973 [22], where colleagues succeeded in being admitted to a mental
institution by pretending to hear a voice saying one word. These pseudo
patients were later released with a diagnosis of “schizophrenia in remission”
[23]. In light of these damning evaluations, Robert Spitzer criticized these
studies as pseudoscience, calling them “logic in remission” [23]. Working with
a Washington University group, Spitzer [23] attempted to consolidate the diagnostic
criteria through the Research Diagnostic Criteria (RDC). RDC was initially a
more reliable set of criterion that had both inclusion and exclusion criteria
[24]. Certain expressions excluded a patient from a diagnosis while other
expressions increased the likelihood of a specific diagnosis. The DSM-III began
to rely on RDC and started describing categories in more detail including
demographic profile of patients, how to differentiate the target category from
similar categories, and a brief discussion of what was known, if anything,
about the course and onset of the disorder. This greater contextual detail was
also supported by evaluations on a broader array of functionality of the
patient. In addition, the DSM-III contained supplementary materials allowing
clinicians to compare different diagnostic criteria between DSM and ICD and
other details known about the disease. This permeability to input from
practicing clinicians allowed the DSM to improve. But there were still problems
with this classification system.
Clinicians were applying their own archetype
of the disease in diagnosing patients. They were comparing their patient with a
typical case rather than identifying unique features of the clinical expression
in accordance with the DSM [25]. Although clinicians’ evaluations proved
consistent (reliable) they were not identical to either the DSM or ICD systems
a practice that diminished their validity [26,27]. At the same time a more
forceful external classification emerged that was again promoting a more aggressive
biological determinism and influencing the DSM. Similar to the 1972 Research
Diagnostic Criteria (RDC) [24], there was a new version of biological
determinism championed by the then director of the U.S. National Institute of
Mental Health (NIMH) Thomas Insel. The Research Domain Criteria (RDoC) baptism
coincided with the publication of the DSM-5 in 2013, and heralds a radical
diagnostic departure by relying exclusively on biomarkers—biological markers.
The ambition of RDoC was to improve the reliability of classifying diseases. As
such it was not a complete departure from the DSM, but it was a more forceful
push for a biological definition of mental disorder. Although the DSM has
incrementally inched its way to favor biological indicators of disease, with
ICD similarly leaning towards this emphasis, RDoC was by birth exclusively
focused on biological correlates of disease.
The implicit assumption being that
behavioral/mental/clinical disorders are manifestations of
biological/neurological disorders. Negative behavior is neural problems in the
physical system. The argument proposed by RDoC is that by finding the bad
circuits we will be able to fix the problem and to “yield new and better
targets for treatment” [28]. While explicitly demoting the importance of
understanding the disease, it elevates the search for a cure. There are
emerging criticism of this new nosology [8,29,30] but what remains untold is
how RDoC is gaining legitimacy.
RDoC’s biological determinism was promoted by
the success of how easy it was for the public and scientists to believe that
Alzheimer’s disease was determined by biomarkers. The history of Alzheimer’s
disease laid the foundation for a new way of biological determinism that has
not been seen since the height of the eugenics movement in 1923 when the
American Eugenics Society was founded. But this emphasis on biology is
unfounded. There is no evidence that biology exclusively determines the
inception, progression and expression of Alzheimer’s disease or many other
mental disorders. But the illusion was made possible by the acceptance of such
an association—that Alzheimer’s disease is purely a neurological disease
controlled by two “mis”-folded proteins.
PROBLEMS WITH
BIOLOGICAL DETERMINISM
Historically only tenuous evidence separated
Alzheimer’s disease from senile (old age) dementia. Alois Alzheimer’s
observation—shared by many of his contemporary researchers—was that the
biomarkers were not unique either for Alzheimer’s disease or among younger
people. But the plaques and tangles found in the brain of Alzheimer’s patients
were elevated as a unique disease by Emil Kraepelin who was Alois Alzheimer’s
supervisor at the Munich clinic. From its inception, Alzheimer’s disease was
promoted as a unique disease because it promoted biological psychiatry.
Alzheimer’s disease supported the belief that genes and biology determine
behavior—borrowing from eugenics—while old age invariably results in diminished
capacity, a similar disease among young people is triggered by
biology—borrowing from ageism. RDoC further supported the legitimacy of
accepting that the plaques and tangles were indicators of Alzheimer’s disease
without providing any supporting evidence but providing a philosophy, a
metaphysical belief of how disease is caused.
THE CAUSES OF
ALZHEIMER’S DISEASE
We continue to ignore our “…incomplete
understanding of AD pathogenesis, the multifactorial etiology and complex
pathophysiology of the disease, the slowly progressive nature of AD
(Alzheimer’s disease) and the high level of comorbidity occurring in the
elderly population” [31]. Arnold pick more than a century ago indicated that “a
mosaic of circumscribed neuropsychological deficits” could cause dementia [32,
p.525]. There are many events that we know cause dementia and/or Alzheimer’s
disease. Including: viral (HIV/AIDS, herpes simplex virus type I, varicella
zoster virus, cytomegalovirus, Epstein-Barr virus), bacteria (syphilis and
lyme-disease/borrelia), parasites (toxoplasmosis, cryptococcosis and
neurocysticercosis), fungi (Candida collaborator), infections (possibly
prions), and vascular (stroke, multiple-infarct dementia, hydrocephalus, injury
and brain tumors) [11,33]. There are other processes that either promote or
delay the infection and the spread of infection, primarily through the
blood-brain-barrier [34], inflammation, vascular, white matter [35] and many
other dynamic processes in the brain. Such models already exist [36]. In
particular understanding how the brain protects itself from getting infected,
and once infected has methods to cope with the infection is an important aspect
of neuropathological development. Protective factors include cognitive reserve
and the capacity of the brain to absorb trauma (maybe including education,
multilingual, exercise, diet, enriched environment in infancy) [37,38]. While
factors that worsen resilience possibly includes: behavior (alcohol, cigarette
smoking, recreational drugs, concussion), environmental elements (possibly
aluminum), and emotional trauma (divorce, death of a loved one, sexual,
physical and emotional abuse and depression) [11]. There are also cascading
effects where one infection destroys or diminishes the ability of another
system to protect the brain. For example, both amyloids and tangles diminish
the blood-brain barrier and thereby expose the brain to outside infections
[39-41]. Such complexity does not beckon simple interventions and does not
easily translate to crowdfunding appeals.
THE SOLUTION
Scientifically, the methodology for studying
Alzheimer’s disease requires a framework that establishes all parameters that
impact the disease; including biological, chemical, neural, clinical,
psychological, social and demographic. These parameters must then be examined
to understand how they interact with each other and within the living
environment (e.g. diet, exercise, stress, work, etc.) [42]. All these
components must be summarized into a coherent theory (as much as is possible.)
From this theory hypotheses can be generated and then tested that have the
capacity to refute the theory.
More importantly the clinical expression of
the disease needs to remain central, as dementia is first and foremost a
clinical disease. If the neuropathology had no clinical outcomes (people do not
express the disease and there is no change in their behavior) then there is no
reason to cure the disease. Rather than focusing on neuroscience and the
biological validity of diagnosis, emphasis needs to be redirected by
recognizing clinicians as worthwhile and informative sources of information. Although
complicated, it behooves us to appreciate that all psychiatric diagnostic tools
are negotiated and malleable [43] and within this process it is imperative to
acknowledge the role philosophical discourse plays in the development of a
classification of disorders including Alzheimer’s disease The lesson learned
from the impressive clinical work of William Langston in understanding and
ultimately developing interventions for Parkinson’s disease provides an apt
lesson [44]. In his review of the history of how he discovered part of the
process of Parkinson’s disease he writes: “Finally, I would like to conclude
with some closing thoughts: If there is an overarching lesson from this story
for clinicians, it is to never forget the power of clinical observation” [45,
p.S16]. But in contrast to this wisdom, research on Alzheimer’s disease, as
dictated by the Framework [4] and by the U.S. federal funding mechanisms at
least, is being pushed towards a more biological determinism discounting good
clinical work. Both historical precedence as well as current conflicts of
interests in Alzheimer’s disease research has muted this lesson [42].
LACK OF CLINICAL
OVERSIGHT
The lack of clinical oversight has created
some disconnect in research. Although alternate theories exist, they remain
ignored [11,46,47] Research remains disorganized, clinicians remain confused,
and the public has become increasingly worried [37,48].
That the biology contributes to and is part
of the process of Alzheimer’s disease is universally agreed upon. However no
universal standards on biomarkers density and cutoff points have been defined
and “…have not yet been established” [4, p. 551]. We do not know if a large
concentration of these biological markers is needed to define a disease or just
a few. Heiko Braak in 2011 after dissecting 2,332 brains ranging in age from 1
to 100 found that only 10 cases had complete absence of Alzheimer’s disease
related biology. Every person over 25 years of age had Alzheimer’s disease
biomarkers [49], therefore it is not logical to assume that these biological
markers cause the disease as some people have the biomarkers and not the
disease. Such inconsistencies are reflected in unexpectedly high false
positives and false negatives—missing identifying those with dementia and
wrongly identifying unimpaired individuals as having dementia.
The authors of the Framework themselves
highlight the unreliability of the definition: “Up to 60% of CU [cognitive
unimpaired] individuals over age 80 years have AD [Alzheimer’s disease]
neuropathologic changes at autopsy or by biomarkers…Thus, using a clinical
diagnosis of ‘AD’ to ascertain absence of disease is associated with an error
rate exceeding 50% in the elderly” [4, p. 552] And then there are false
negatives, where the majority of people with Alzheimer’s disease do not show
any of the biomarkers. This observation by itself refutes the theory. Even the
authors acknowledge these false negative cases “…using a clinical diagnosis of
'AD’ to ascertain absence of disease is associated with an error rate exceeding
50% in the elderly” [4, p. 552]. There is no scientific precedence for adopting
a definition of a disease that relies on the probability of a coin toss [42].
The main motive for the framework was to
develop strategies for a cure. “This approach also will enable a more precise
approach to interventional trials where specific pathways can be targeted in
the disease process and in the appropriate people” [4, p. 536]. Science is not
beholden to outcomes. Science is a method of acquiring knowledge and a method
cannot determine the outcome of the knowledge gathered. Engineering an outcome
is not science but applied science or business application. Even in the
pharmaceutical business, the industry itself acknowledges that there are other
problems with Alzheimer’s disease other than a cure. In the forward to the 2018
report on Alzheimer’s disease research by the pharmaceutical industry George
Vradenburg with US Against Alzheimer’s writes “…there is a shortage of
geriatricians to care for the country’s aging population, patients are commonly
misdiagnosed, there continue to be long wait times to see neurologists, racial
disparities persist and many patients are never told of their diagnosis by
their doctor” [50].
FEDERAL FUNDING
Despite that the 99% failure rate of
Alzheimer’s disease drug development [51] with a 100% failure rate of
disease-modifying therapies for Alzheimer’s disease [52] in 2014, the
G8—France, Germany, Italy, Japan, United Kingdom, United States, Canada and
Russia—stated that dementia should be made a global priority with the aim of a
cure or treatment by 2025 [53]. In contrast, in 2018 Pfizer, the world’s third
largest drug maker announced that it is ending research in Alzheimer’s disease.
In the past 20 years, Pfizer has conducted over a hundred clinical trials,
testing twenty-four potential Alzheimer’s drugs resulting in only one drug,
Aricept, being approved.
The reality is that Alzheimer’s drugs are
very expensive and so far proved ineffective. Estimates suggest that the cost
of one new drug is now $5.7 billion [54] Funding for such exuberant failures is
primarily through federal finance which for Alzheimer’s disease is through a
network of federal agencies under the umbrella of the National Institutes of
Health (NIH). These interagency funding includes the National Institute on
Aging, National Institute of Mental Health, National Institute of General
Medical Sciences, and National Center for Advancing Translational Science. In
addition, other federal agencies such as the National Science Foundation,
Veterans Administration, Food and Drug Administration, and the Center for
Medicare and Medicaid Services all provide additional funding in Alzheimer’s
disease research. In 2018, the NIH’s spending on Alzheimer’s and related
dementias research was estimated at $1.9 billion. With the 2019 budget targets
including an additional $425 million [55] and is now nearly equal to funding
for cardiovascular disease the main killer in developed countries but still
below funding for cancer. But there are other funds that go into this expanding
research pot. Other inter- and intra-agency collaborations have separate
funding mechanisms for Alzheimer’s disease beyond NIH, including private
equity, research organizations, not-for-profit advocacy and philanthropic
organizations, academic institutions, pharmaceutical companies and individual
State funding sources [52]. New sources of funding are now being aimed at
tapping public support through crowdfunding [56]. Sources of funding for
Alzheimer’s disease are similarly diverse in Europe. The United Kingdom has
just funded a new initiative Dementia Discovery Fund with £250 million ($327
million) while the European Union funded three Alzheimer’s disease Research
Platform projects from the Innovative Medicines Initiative with €138 million
($154 million).
Alzheimer’s disease research is already one
of the top medical research concerns worldwide, and funding is slated to grow.
But as Hayflick [57] comments on these budgetary successes, with all this money
why not focus on the biology of aging rather than on piecemeal studies on
Alzheimer’s disease. He comments “What would be more important than a budget
increase that favors research on Alzheimer's disease and other age-related
disease is to focus on research on the etiology of biological aging.”
WHAT ARE WE TRYING
TO CURE?
Alzheimer’s disease mainly afflicts older
adults although the disease was initially diagnosed explicitly in younger
people. The merger occurred when one of the founders of the National Institute
on Aging, Katzman [58]—in an effort to gain funding for the establishment of
the NIA in the 1970s—combined the rare Alzheimer’s disease with the much higher
prevalence of senile dementia. Katzman admitted that the numbers of “pure”
Alzheimer’s disease were so small that “Precise epidemiological information [on
Alzheimer’s disease] is not available…” [58, p.378]. With this trick of
combining Alzheimer’s disease with senile (old-age) dementia Katzman [58]
announced in the title of his paper that Alzheimer’s disease is a “major
killer” in the USA. Such dramatic admissions hide some technical difficulties.
Alzheimer’s disease among older adults captures other diseases in the
diagnosis. Older adults confront a cumulative number of diseases as they age.
Some of these diseases have been found to contribute or at least accompany the
development of Alzheimer’s disease, such as hypertension, arteriosclerosis,
depression, anxiety and a host of vascular diseases [59]. Alzheimer’s disease
in isolation from these other chronic diseases is rare and among older adults
unlikely and under-reported [60]. In one large study only 0.01% of patients had
a diagnosis of dementia with no co-morbid conditions [61]. It is rare for older
adults to have brain disease in isolation from other type of (non-cognitive)
diseases such as depression [62] and anxiety [63]. Since individuals have
multiple comorbidities, isolating the disease includes both a clinical problem
as well as a neurological one [64]. As a result, among older people, many
dementias are misdiagnosed [65-67] This helps explain why multiple studies have
shown that the correlation between plaques and tangles and Alzheimer’s disease
declines with age since there are other factors that are causing cognitive
problems [68]. But such evidence remains what Kuhn calls incommensurable—this
evidence cannot be acknowledged let alone accepted.
The primary theory in Alzheimer’s disease is
presented by the amyloid cascade hypothesis [69]. This theory proposes that
active immunization against amyloid-β42 peptide (plaques) and neurotic tau
(tangles) would cure the disease. So far, all types of immunization trials for
both plaques and tangles continue to fail. The active amyloid immunization
clinical trial by Elan Pharmaceuticals (AN1792) indicated that amyloid can be
cleared from the brain. However cognition was not improved even after long-term
follow-up [70-73]. This suggests that the plaques cannot be causing the disease
[74]. The Framework now argues that the amyloids are precursors to the real
disease that are the tau tangles, an argument made a century ago by Oskar
Fischer [75]. But this strategy adopts the same assumptions as for the amyloid
hypothesis [76] and so far, the results have been predictably insignificant and
diffuse [77,78].
Older people have complex clinical issues.
People will inevitably continue to die and as populations get older, older
people will continue to die at higher numbers. If we eliminated the top
diseases of older adults, such as cancer, diabetes, cardiovascular disease,
stroke, influenza and pneumonia and chronic obstructive lung disease older
people will still die at a slightly older age. There will be a small extension
of life. It seems counterintuitive that by eliminating one disease older people
might experience slightly longer life with more disability. Since older adults
suffer from not just one but multiple health conditions it is only a matter of
time that one disease will prove to be the exist disease. Statistically
eliminating musculoskeletal conditions would result in an additional year of
good health for women and less than half a year for men [79]. But there are
also negative outcomes of curing diseases. By eliminating cardiovascular
disease or cancer a proportion of the years of life gained would be spent in
poorer health and increased cost [79]. While in contrast, eliminating mental
conditions (including depression and suicide) will result in fewer gains in
life expectancy but with reduced periods of illness [80]. In the best-case
scenario, by eliminating all major killer diseases, life expectancy at birth in
2019 will be expected to increase to 96 years [81]. But we will still die. The
aging of population, by itself—with or without Alzheimer’s disease—people will
continue to die at increasing numbers since that population has succeeded at
living longer. In support of Hayflick’s [57] argument, singling out one disease
to cure is as illogical as conducting invasive surgery on moribund patients.
QUALITY OF LIFE
Although we are fearful of dementia, and this
fear seems to be growing [82,83], reflecting our increasing fear of aging [84],
quality of life for people with dementia does not necessarily decrease as the
dementia progresses [85-87]. Although studies show variable and inconsistent
results, there is a common acceptance by social scientists that under certain
circumstances people living with dementia are not necessarily less happy then
they were before the diagnosis. Beerens et al. [88,89] report two studies that
show that three months following admission to a long-term care facility only
those with better cognitive abilities reported a decrease in their quality of
life (they were aware of their reduced capacity). A general trend is that
people with dementia living at home show more depressive symptoms compared to
those living in long term care facilities. In fact, Payne et al. [90] found
that depression is reduced after entering a long-term care facility, which may
reflect on what Kitwood [91] terms as the negative interpersonal dynamics at
home. Kitwood [91] argues that some deterioration is the result of how the
person with dementia is treated rather than by the disease itself. He called
this “malignant social psychology” where a caregiver’s relationship, in some
extreme cases, devalue, dehumanizes and diminishes the person with dementia by
being stigmatized, infantilized, objectified or ignored. In less dramatic
situations however, Alzheimer’s disease is rarely experienced in isolation from
a broader social context.
This interpersonal dynamic is an important
component of life for people living with dementia. In a 2014 longitudinal study
Clare et al. [92] reported that over a 20 month period one-third of people
living with dementia rated their quality of life higher. The determining factor
was the negative quality of the relationship with their caregiver and taking
acetylcholinesterase-inhibiting medication. Caregivers want you to be the
person that you used to be, which is why after 18 months in a long-term
facility, even though self-rating of the quality of life did not change for the
person with dementia their caregivers rated them as less happy [93].
Caregivers’ base their judgment on the patient’s cognitive and
functional/physical decline, but for people living with dementia it was anxiety
that mediated their rating. In most cases, anxiety is promoted by unreachable
expectations from their caregivers. In most cases, by being away from their
caregivers, people living with dementia expressed reduced anxiety and therefore
reported better quality of life [93].
Research indicates that there is no
straightforward relationship between quality of life and dementia. There is
much complexity in social contexts and quality for people for people with
dementia varies consistently by country [88]. For those living in nursing
homes, depression lowered their quality of life whereas for those living at
home, falls reduced their quality of life. There are many confounding factors,
but the evidence is consistent. A year after receiving the devastating
diagnosis of dementia, most patients revert to their previous level of
wellbeing.
It is caregivers that suffer the greatest
loss of reported quality of life, both in terms of their interaction with the
patient and their own health and wellbeing. Caregivers—whether they are still
providing care or those whose care-recipient died or became
institutionalized—all expressed a great amount of psychological distress,
including: depression, anxiety, interpersonal sensitivity and paranoid ideation
and difficulty mental performance [94]. When compared with spouses who were
caring for a spouse without dementia, caregivers of a spouse with dementia had
higher psychological distress [95]. Caregivers’ interaction with their care
receiver determines the quality of life of both. It is the great sorrow that
caregivers feel when their loved-one start to lose who they were. It is this
angst that Crowdfunding appeals to.
CONCLUSION
The potential for crowdfunding in Alzheimer’s
disease is great. You have the perfect storm of anguished family members a
disease that is being promoted as caused by simple biology of two misfolded
proteins, affecting nearly everyone directly or indirectly, and there is great
hype that a cure is around the corner. Combined with the difficulty for new
researchers to get into the federal funding stream because of a cabal of
researchers and their ever expanding research institutes, the constant failure
rate of ongoing disease-modifying interventions and the increasing fear in the
media all lead to the false impression that not enough funding is devoted to
Alzheimer’s disease research while at the same time a cure is just around the
corner. Crowdfunding has the potential to fulfill a gap in this perceived
funding gap. But using crowdfunding for research promotes pseudo-science [96].
Crowdfunding relies on emotional rather than scientific arguments. The fear of
Alzheimer’s disease will drive the urgency of such appeals. They are reliant on
people’s need for binary answers when, as discussed, there is great complexity
in the disease. This is at a time when crowdfunding for science has become more
attractive for younger researchers in academic institutions. More than 1,000
medical crowdfunding campaigns for 5 treatments that are unsupported by
evidence or are potentially unsafe have raised more than $6.7 million [97].
While 408 campaigns raised more than $1 million for unproven stem cell
interventions [59].
While established researchers in Alzheimer’s
disease have an invested interest in maintaining adherence to a simplified but
defunct theory, emerging researchers have very few options for funding.
Although U.S. federal funding is increasing for Alzheimer’s disease research,
as are other sources of funding, there is a lack of diversity in funding
recipients (especially for diverse approaches). Crowdfunding will seem as a
solution. But given the nuances of a disease that interferes with the brain—one
of the most complex organs ever encountered—translating the problem into a
venture capital issue dummies down the complexity and diminishes the likelihood
that the right approach will be taken. The overall problem is that such nuanced
approach to research requires strong federal support. Big science requires big
funding support. However changing the direction within the U.S. federal health
funding mechanism requires a revolution. Kuhn was right in highlighting the
social aspect of science we now need to admit to this dimension in or work and
address it before we waste another 70 years of research on a theory that has
outlived its utility. Addressing dementia will require this level of political
commitment.
1. Popper
K (2014) Conjectures and refutations: The growth of scientific knowledge.
Routledge.
2. Kuhn
TS (2012) The structure of scientific revolutions. University of Chicago Press.
3. Sauermann
H, Franzoni C, Shafi K (2018) Crowdfunding scientific research. National Bureau
of Economic Research.
4. Jack
CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, et al. (2018) NIA-AA Research
Framework: Toward a biological definition of Alzheimer's disease. Alzheimers
Dement 14: 535-562.
5. Fu C,
Chute DJ, Farag ES, Garakian J, Cummings JL, et al. (2004) Comorbidity in
dementia: An autopsy study. Arch Pathol Lab Med 128: 32-38.
6. Strobel
G (2007) Alzheimer research forum report: Tübingen: The man behind the eponym.
J Alzheimers Dis 11: 131-133.
7. Weuve
J, Proust-Lima C, Power MC, Gross AL, Hofer SM, et al. (2015) Guidelines for
reporting methodological challenges and evaluating potential bias in dementia
research. Alzheimers Dement 11: 1098-1099.
8. Jessen
F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, et al. (2014) A
conceptual framework for research on subjective cognitive decline in
preclinical Alzheimer's disease. Alzheimers Dement 10: 844-852.
9. Weinberger
DR, Glick ID, Klein DF (2015) Whither research domain criteria (RDoC)? The
good, the bad and the ugly. JAMA Psychiatry 72: 1161-1162.
10. Au R,
Piers RJ, Lancashire L (2015) Back to the future: Alzheimer's disease
heterogeneity revisited. Alzheimers Dement (Amst) 1: 368-370.
11. Snyder
J, Turner L, Crooks VA (2018) Crowdfunding for unproven stem cell-based
interventions. JAMA 319: 1935-1936.
12. Garrett
MD, Valle R (2015) A new public health paradigm for Alzheimer's disease
research. SOJ Neurol 2: 1-9.
13. Jack
Jr CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, et al. (2011)
Introduction to the recommendations from the National Institute on
Aging-Alzheimer's Association workgroups on diagnostic guidelines for
Alzheimer's disease. Alzheimers Dement 7: 257-262.
14. Jack
CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, et al. (2018) NIA-AA Research
Framework: Toward a biological definition of Alzheimer's disease. Alzheimers
Dement 14: 535-562. Supplemental Material accessed: https://www.alzheimersanddementia.com/cms/attachment/2119162008/2089988545/mmc1.docx
15. Lenzer
J (2011) French guidelines are withdrawn after court finds potential bias among
authors. BMJ 2011: 342.
16. Lehmann
HE, Ban TA (1997) The history of the psychopharmacology of schizophrenia. Can J
Psychiatry 42: 152-162.
17. Kleinman
A (1988) Rethinking psychiatry: From cultural category to personal experience.
New York: Free Press.
18. Blashfield
RK, Keeley JW, Flanagan EH, Miles SR (2014) The cycle of classification: DSM-I
through DSM-5. Annu Rev Clin Psychol 10: 25-51.
19. Decker
HS (2013) The making of DSM-III: A diagnostic manual's conquest of American
psychiatry. Oxford University Press.
20. Yasgur
BS (2018) Push is on to reclassify schizophrenia as a neurologic disease.
Medscape.
21. Kendell
RE, Cooper JE, Gourlay AJ, Copeland JR, Sharpe L, et al. (1971) Diagnostic
criteria of American and British psychiatrists. Arch Gen Psychiatry 25:
123-130.
22. Rosenhan
DL (1973) On being sane in insane places. Science 179: 250-258.
23. Spitzer
RL, Endicott J, Robins E (1975) Clinical criteria for psychiatric diagnosis and
DSM-III. Am J Psychiatry 132: 1187-1192.
24. Feighner
JP, Robins E, Guze SB, Woodruff RA, Winokur G, et al. (1972) Diagnostic
criteria for use in psychiatric research. Arch Gen Psychiatry 26: 57-63.
25. Livesley
WJ (1986) Trait and behavioral prototypes of personality disorder. Am J
Psychiatry 143: 728-732.
26. Reed
GM, Roberts MC, Keeley J, Hooppell C, Matsumoto C, et al. (2013) Mental health
professionals' natural taxonomies of mental disorders: implications for the
clinical utility of the ICD_11 and the DSM_5. J Clin Psychol 69: 1191-212.
27. Roberts
MC, Reed GM, Medina-Mora ME, Keeley JW, Sharan P, et al. (2012) A global
clinicians' map of mental disorders to improve ICD-11: Analysing meta-structure
to enhance clinical utility. Int Rev Psychiatry 24: 578-590.
28. Insel
T (2013) Director's blog: Transforming diagnosis. Accessed: http://www.nimh.nih.gov/about/director/2013/transforming-diagnosis.shtml
29. Nemeroff
CB, Weinberger D, Rutter M, MacMillan HL, Bryant RA, et al. (2013) DSM-5: A
collection of psychiatrist views on the changes, controversies and future
directions. BMC Med 11: 202.
30. Peterson
BS (2015) Research Domain Criteria (RDoC): A new psychiatric nosology whose
time has not yet come. J Child Psychol Psychiatry 56: 719-722.
31. Sugino
H, Watanabe A, Amada N, Yamamoto M, Ohgi Y, et al. (2015) Global trends in
Alzheimer disease clinical development: Increasing the probability of success.
Clin Ther 37: 1632-1642.
32. Spatt
J (2003) Arnold Pick's concept of dementia. Cortex 39: 525-531.
33. Garrett
MD (2015) Politics of Anguish: How Alzheimer's disease became the malady of the
21st century. CreateSpace.
34. Deane
R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-peptide across
the blood-brain barrier: Implication for therapies in Alzheimer's disease. CNS
Neurol Disord Drug Targets 8: 16-30.
35. Serrano-Pozo
A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, et al. (2011)
Reactive glia not only associates with plaques but also parallels tangles in
Alzheimer's disease. Am J Pathol 179: 1373-1384.
36. Schelke
MW, Attia P, Palenchar D, Kaplan B, Mureb M, et al. (2018) Mechanisms of risk
reduction in the clinical practice of Alzheimer's disease prevention. Front
Aging Neurosci 10: 96.
37. Garrett
MD, Valle R (2016) A century of confusion in researching Alzheimer's disease.
Int J Healthcare 2: 13.
38. Garrett
MD, Valle R (2016) A methodological critique of the National Institute of Aging
and Alzheimer's Association Guidelines for Alzheimer's disease, dementia and
mild cognitive impairments. Dementia 15: 239-254.
39. Bell
RD, Zlokovic BV (2009) Neurovascular mechanisms and blood-brain barrier
disorder in Alzheimer's disease. Acta Neuropathologica 118: 103-113.
40. Shibata
M, Yamada S, Kumar SR, Calero M, Bading J, et al. (2000) Clearance of
Alzheimer's amyloid-1-40 peptide from brain by LDL receptor-related protein-1
at the blood-brain barrier. J Clin Investig 106: 1489-1499.
41. Zenaro
E, Piacentino G, Constantin G (2017) The blood-brain barrier in Alzheimer's
disease. Neurobiol Dis 107: 41-56.
42. Garrett
MD (2018) A Critique of the 2018 National Institute on Aging's. Research
Framework: Toward a biological definition of Alzheimer's disease. 2018: 49-58.
43. Sadler
JZ (2005) Values and psychiatric diagnosis. Oxford University Press.
44. Langston
JW, Palfreman J (2013) The case of the frozen addicts: How the solution of a
medical mystery revolutionized the understanding of Parkinson's disease? IOS
Press.
45. Langston
JW (2017) The MPTP story. J Parkinsons Dis 7: S11-19.
46. Whitehouse
PJ (2014) The end of Alzheimer's disease - From biochemical pharmacology to
ecopsychosociology: A personal perspective. Biochem Pharmacol 88: 677-681.
47. The
LN (2016) Finding a cure for Alzheimer's disease starts with prevention. Lancet
Neurol 15: 649.
48. Ballenger
JF (2017) Framing confusion: Dementia, society and history. AMA J Ethics 19:
713-719.
49. Braak
H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic
process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol
Exp Neurol 70: 960-969.
50. PHRMA
(2019) Researching Alzheimer's disease: Setbacks and stepping stones. Accessed:
http://phrma-docs.phrma.org/files/dmfile/AlzheimersSetbacksSteppingStones_FINAL_digital.pdf
51. Cummings
JL, Morstorf T, Zhong K (2014) Alzheimer's disease drug-development pipeline:
few candidates, frequent failures. Alzheimers Res Ther 6: 37.
52. Cummings
J, Reiber C, Kumar P (2018) The price of progress: Funding and financing
Alzheimer's disease drug development. Alzheimers Dement Transl Res Clin Interv
4: 330-343.
53. Vradenburg
G (2015) A pivotal moment in Alzheimer's disease and dementia: How global unity
of purpose and action can beat the disease by 2025. Expert Rev Neurother 15:
73-82.
54. Scott
TJ, O'connor AC, Link AN, Beaulieu TJ (2014) Economic analysis of opportunities
to accelerate Alzheimer's disease research and development. Ann N Y Acad Sci
1313: 17-34.
55. USNIA
(2019) Richard Hodes: NIA we have a budget for FY 2019! NIH Research Blog.
Accessed: https://www.nia.nih.gov/research/blog/2018/10/we-have-budget-fy-2019
56. Carter
AJ, Donner A, Lee WH, Bountra C (2017) Establishing a reliable framework for
harnessing the creative power of the scientific crowd. PLoS Biol 15: e2001387.
57. Hayflick
L (2019) Comment. In: Richard Hodes: NIA We have a budget for FY 2019. NIH
2019. Accessed: https://www.nia.nih.gov/research/blog/2018/10/we-have-budget-fy-2019
58. Katzman
R (1976) The prevalence and malignancy of Alzheimer disease: A major killer.
Arch Neurol 33: 217-218.
59. Snyder
HM, Asthana S, Bain L, Brinton R, Craft S, et al. (2016) Sex biology
contributions to vulnerability to Alzheimer's disease: A think tank convened by
the women's Alzheimer's research initiative. Alzheimers Dement 12: 1186-1196.
60. Doraiswamy
PM, Leon J, Cummings JL, Marin D, Neumann PJ (2002) Prevalence and impact of
medical comorbidity in Alzheimer's disease. J Gerontol Series A Biol Sci Med
Sci 57: M173-177.
61. Sanderson
M, Wang J, Davis DR, Lane MJ, Cornman CB, et al. (2002) Co-morbidity associated
with dementia. Am J Alzheimers Dis Other Demen 17: 73-78.
62. Wagner
GS, McClintock SM, Rosenquist PB, McCall WV (2011) Major depressive disorder
with psychotic features may lead to misdiagnosis of dementia: A case report and
review of the literature. J Psychiatr Pract 17: 432.
63. Guziak
CC, Smith JE (2014) Anxiety misdiagnosed as dementia? A complex case
successfully treated using a multimodal biofeedback approach. Biofeedback 42:
12-15.
64. Qiu
C, De Ronchi D, Fratiglioni L (2007) The epidemiology of the dementias: An
update. Curr Opin Psychiatry 20: 380-385.
65. Nielsen
TR, Andersen BB, Kastrup M, Phung TK, Waldemar G (2011) Quality of dementia
diagnostic evaluation for ethnic minority patients: A nationwide study. Dement
Geriatr Cogn Disord 31: 388-396.
66. Black
S, Simpson GM (2014) A call to action: Dementia screening of Alzheimer's
disease in older African Americans. In: The collective spirit of aging across
cultures 2014. Springer: Dordrecht, pp: 229-238.
67. Sayegh
P, Knight BG (2013) Assessment and diagnosis of dementia in Hispanic and
non-Hispanic white outpatients. Gerontol 53: 760-769.
68. Savva
GM, Wharton SB, Ince PG, Forster G, Matthews FE, et al. (2009) Age,
neuropathology and dementia. N Engl J Med 360: 2302-2309.
69. Hardy
JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis.
Science 256: 184-186.
70. Hock
C, Konietzko U, Streffer JR, Tracy J, Signorell A, et al. (2003) Antibodies
against amyloid slow cognitive decline in Alzheimer's disease. Neuron 38:
547-554.
71. Bayer
AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, et al. (2005) Evaluation of
the safety and immunogenicity of synthetic A_42 (AN1792) in patients with AD.
Neurology 64: 94-101.
72. Gilman
S, Koller M, Black RS, Jenkins L, Griffith SG, et al. (2005) Clinical effects
of A_immunization (AN1792) in patients with AD in an interrupted trial.
Neurology 64: 1553-1562.
73. Holmes
C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, et al. (2008) Long-term
effects of A_42 immunisation in Alzheimer's disease: follow-up of a randomised,
placebo-controlled phase I trial. Lancet 372: 216-223.
74. Iqbal
K, Liu F, Gong CX (2014) Alzheimer disease therapeutics: Focus on the disease
and not just plaques and tangles. Biochem Pharmacol 88: 631-639.
75. Goedert
M (2008) Oskar Fischer and the study of dementia. Brain 132: 1102-1111.
76. Cappa
SF (2018) The quest for an Alzheimer therapy. Front Neurol 9: 108.
77. Boche
D, Donald J, Love S, Harris S, Neal JW, et al. (2010) Reduction of aggregated
Tau in neuronal processes but not in the cell bodies after A_42 immunisation in
Alzheimer's disease. Acta Neuropathologica 120: 13-20.
78. Li X,
Kaida-Yip F, Zabel M (2018) NSAID use and the prevention of Alzheimer's
disease: A meta-analysis (P6.184). Neurology 90.
79. Manuel
DG, Schultz SE, Kopec JA (2002) Measuring the health burden of chronic disease
and injury using health adjusted life expectancy and the health utilities
index. J Epidemiol Community Health 56: 843-850.
80. Tanuseputro
P, Manuel DG, Leung M, Nguyen K, Johansen H (2003) Risk factors for
cardiovascular disease in Canada. Can J Cardiol 19: 1249-1260.
81. Manton
KG, Patrick CH, Stallard E (1980) Mortality model based on delays in
progression of chronic diseases: Alternative to cause elimination model. Public
Health Rep 95: 580.
82. Interactive
H (2006) MetLife Foundation Alzheimer's survey: What America thinks. A MetLife
Foundation commissioned report.
83. MIPO-Marist
Institute for Public Opinion (2012) Alzheimer's most feared disease survey for
Home Instead Senior Care. Accessed: http://www.helpforalzheimersfamilies.com/alzheimers-dementia-care-services/alzheimers_feared_disease/
84. O'rourke
N (1996) Alzheimer's disease as a metaphor for contemporary fears of aging. J
Am Geriatr Soc 44: 220-221.
85. Selwood
A, Thorgrimsen L, Orrell M (2005) Quality of life in dementia - A one year
follow-up study. Int J Geriatr Psychiatry 20: 232-237.
86. Hoe
J, Hancock G, Livingston G, Woods B, Challis D, et al. (2009) Changes in the
quality of life of people with dementia living in care homes. Alzheimer Dis
Assoc Disord 23: 285.
87. Bosboom
PR, Alfonso H, Almeida OP (2013) Determining the predictors of change in
quality of life self-ratings and carer-ratings for community-dwelling people
with Alzheimer disease. Alzheimer Dis Assoc Disord 27: 363-371.
88. Beerens
HC, Sutcliffe C, Renom-Guiteras A, Soto ME, Suhonen R, et al. (2014)
RightTimePlaceCare Consortium. Quality of life and quality of care for people
with dementia receiving long term institutional care or professional home care:
The European RightTimePlaceCare study. J Am Med Directors Assoc 15: 54-61.
89. Beerens
HC, Zwakhalen SM, Verbeek H, Ruwaard D, Ambergen AW, et al. (2015) Change in
quality of life of people with dementia recently admitted to long-term care
facilities. J Adv Nurs 71: 1435-1447.
90. Payne
JL, Sheppard JM, Steinberg M, Warren A, Baker A (2002) Incidence, prevalence
and outcomes of depression in residents of a long-term care facility with
dementia. Int J Geriatr Psychiatry 17: 247-253.
91. Kitwood
T (1998) Toward a theory of dementia care: Ethics and interaction. J Clin
Ethics 9: 23-34.
92. Clare
L, Woods RT, Nelis SM, Martyr A, Markova IS, et al. (2014) Trajectories of
quality of life in early stage dementia: Individual variations and predictors
of change. Int J Geriatr Psychiatry 29: 616-623.
93. Bosboom
PR, Alfonso H, Almeida OP (2013) Determining the predictors of change in
quality of life self-ratings and carer-ratings for community-dwelling people
with Alzheimer disease. Alzheimer Dis Assoc Disord 27: 363-371.
94. Pot
AM, Deeg DJ, Van Dyck R (1997) Psychological well-being of informal caregivers
of elderly people with dementia: changes over time. Aging Ment Health 1:
261-268.
95. Ask
H, Langballe EM, Holmen J, Selbæk G, Saltvedt I, et al. (2014) Mental health
and wellbeing in spouses of persons with dementia: The Nord-Trøndelag health
study. BMC Public Health 14: 413.
96. Newman
M (2018) Is cancer fundraising fuelling quackery? BMJ 362: 3829.
97. Vox
F, Folkers KM, Turi A, Caplan AL (2018) Medical crowdfunding for scientifically
unsupported or potentially dangerous treatments. JAMA 320: 1705-1706.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)