500
Views & Citations10
Likes & Shares
REGENERATIVE
MEDICINE
It
is the field of research and clinical applications focused on the repair and
regeneration of cells, tissues or organs in order to restore a damaged
function. New techniques in regenerative medicine using the “Stromal Vascular
Fraction” (SVF) of the perichondrium of the patient's own atrial cartilage [1].
The
vascular stromal fraction is formed by different cells, with the capacity to
regenerate those tissues damaged both by trauma and by the aging and cellular
wear. In the SVF of the perichondrium of the auricular cartilage, we find large
numbers and viability of cells to generate new adipose tissue and blood vessels
as well as produce growth factors that and to the formation of the vascular
network [2,3].
CHARACTERISTICS OF
THE SVF
·
Ability to differentiate in different
lineages and are very useful for tissue replacement therapy (refers to
multipotentiality) [4,5].
·
They can be administered directly in the
affected area where tissue regeneration is attempted or
systemically/intravenously [6].
·
They can secrete soluble factors that
promote the paracrine effect and immunomodulators that facilitate the
therapeutic effects [7,8].
·
They are immunoprivileged that allows a
minimal immune reaction due to the lack of expression of class II
immunocompatibility. They present receptors and can be directed or migrate to
the places of the lesion [9].
·
When fresh ADSCs are isolated, a
non-cultured heterogeneous population is obtained, which are the SVF (Vascular
Stromal Fraction) cells with therapeutic qualities that are better adapted to
the different clinical scenarios.
The principle on which this technology is
based is to use healthy counterpart connective tissue of the same patient
processed with KIT to regenerate its own damaged tissue.
The affinity of the donor and recipient
tissue used contributes a high differentiation and potentiality obtaining as a
result a great cellular regenerative efficiency.
The lumbar disc herniation is the
result of a protrusion or prolapse of the nucleus pulposus of an intervertebral
disc in the spinal canal, after perforation and partial or complete destruction
of the posterior part of the annulus fibrosis. When this happens, the nerve
root is compressed by the nucleus pulposus, causing various symptoms, such as
pain in the lower limb, back pain and feeling of wheezing.
CLINICAL CASE
We know the
different treatments of the pathology of the Lumbar disc herniation; we
performed the first lumbar disc pathology treatment with the tissue micrograft
technique in the nucleus pulposus, in a patient with a contained disc
Herniation (fibrous ring without rupture).
Patients
aged between 18 and 70 years, with degenerative disease: (Disc prothesis: black
disc) with predominant back pain after conservative treatment (physical and
medical) for more than 6 months. Patients must have a fibrous ring capable of
supporting the cellular implantation; as shown by the MRI image (exclusion of
extruded material).
The technique consists:
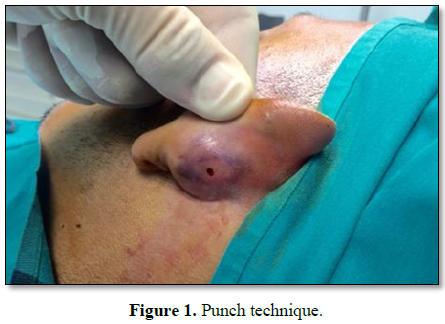
With local anesthesia a 2.5 mm
punch of the auricular cartilage (Figure
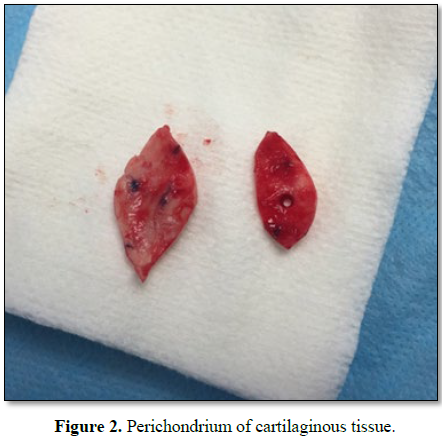
1), containing articular cartilage perichondrium, is obtained (Figure 2).
The PERICONDRIO is constituted by dense
connective tissue and covers the cartilages except for the free surface of the
articular face. Its main functions are, to nourish the cartilage through the
blood vessels, eliminate the waste products and of originating new
chondroblasts from chondrogenic cells of its inner layer.
The extraction of cartilage is done with the
punch making movements soft rotating to release cartilage but never going
through the skin of the posterior zone.
When extracting the micrograft from the
punch, the punch is rotated downwards to release the cartilage, in the case
that do not release, you would have to remove the cartilage with perichondrium,
introducing a clamp in the hole.
The sample obtained must contain skin,
perichondrium and cartilage; also it must be cylindrical, white and
cartilaginous in appearance.
If there is bleeding, hemostasis can be done
with different methods, the most used is mechanical pressure with sterile gauze
made by a helper or the same patient. If the bleeding still persists, it will
be used a fine-tipped clamp and electrocoagulation of the holes made by the
punch (no surgical suture is needed, as it will heal by the second intention in
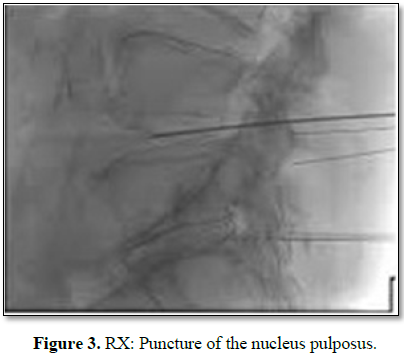
a week) once obtained the SVF and through the use of a needle the content is
injected into the nucleus pulposus, following the same technique that is used
in nucleoplasty and percutaneous techniques (Figure 3).
Being an ambulatory
technique; after a rest of about 30 min, the patient is discharged with the
recommendations of the immediate postoperative period. So it is convenient that
the patient take, if necessary, analgesics, but never anti-inflammatory pills
as they can stop the process. Regarding rest, relative rest is recommended,
allowing the patient to walk progressively for about 7 days. After this period,
you can start doing physical exercise and rehabilitation physiotherapy. When the
ear heals, swimming exercises can also be done.
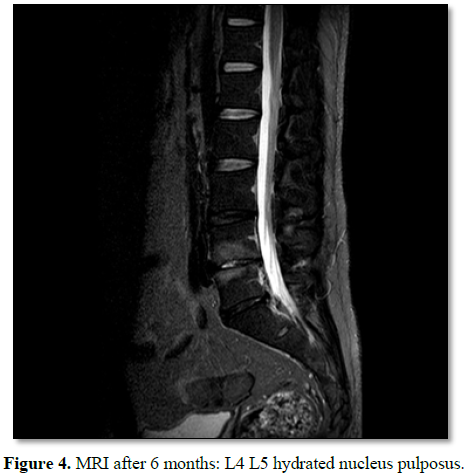
The pathology
identical to that performed in any percutaneous lumbar procedure is monitored (Figure
4).
DISCUSSION
The main treatment for lumbar disc herniation
is conservative and the response is approximately 80%. Conservative treatment
includes rest, bed rest, pharmacological treatment (e.g. non-steroidal
anti-inflammatory drugs, corticosteroids and muscle relaxants), use of a
corset, traction therapy, thermotherapy, epidural block, nerve root block and
physiotherapy. Between 20% and 50% of the patients, approximately, are
tributaries of surgical treatment (when no improvement is observed with a
conservative treatment) [10].
To reduce the invasiveness of surgical
procedures, new therapeutic approaches have emerged, including
chemonucleolysis, percutaneous nucleotomy, percutaneous laser disc
decompression and microendoscopic discectomy.
Degenerative disc disease (DDD) is a
condition associated with the degeneration of one or more of the discs in the
spine. DDD can cause severe chronic pain in the back at the level of the disc
region and it can radiate to the hips and legs. There may be severe
inflammation and degeneration of the fibrocartilage.
The perichondrium of the auricular cartilage
is a new therapy for patients with degenerative disc disease. SVF injected
directly into the disc can reduce inflammation and promote healing. SVF is an
attractive therapeutic method since the collection process is safe and the
cells are readily available in generally large quantities.
Clinical studies have demonstrated the safety
and feasibility of using SVF in patients with degenerative disc. No major
safety problems were observed and the procedures were well tolerated in all
patients. In addition, patients showed statistically significant improvements
in several parameters including flexion, pain classifications, VAS, PPI and
questionnaires in abbreviated form. Although ODI and BDI did not show
statistically significant changes due to the low number of subjects in the
trial, the data allow verifying positive trends. In addition, most patients
reported improvements in their Dallas Pain Questionnaire scores.
CONCLUSION
This percutaneous technique of regenerative
medicine is beginning in our unit and our desire is to carry out prospective
studies to make the comparison with other known techniques of the percutaneous
type and see if the results are significant to be used within the existing
techniques.
1. Moseley
TA, Zhu M, Hedrick MH (2006) Adipose-derived stem and progenitor cells as
fillers in plastic in reconstructive surgery. Plast Reconstr Surg 118:
121S-128S.
2. Matsumoto
D, Shigeura T, Sato K, Inoue K, Suga H, et al. (2007) Influences of
preservation at various temperatures on liposuction aspirates. (Plast Reconstr
Surg 120: 1510-1517.
3. Suga
H, Matsumoto D, Inoue K, Shigeura T, Eto H, et al. (2008) Numerical measurement
of viable and nonviable adipocytes and other cellular components in aspirated
fat tissue. Plast Reconstr Surg 122: 103-114.
4. Charles-de-Sá
L, Gontijo-de-Amorim NF, Maeda Takiya C, Borojevic R, Benati D, et al. (2015)
Anti-aging treatment of the facial skin by fat graft and adipose-derived stem
cells. Plast Reconstr Surg 135: 999-1009.
5. Gimble
JM, Guilak F (2003) Adipose-derived adult stem cells: Isolation,
characterization and differentiation potential. Cytotherapy 5: 362.
6. Hauner
H (1989) Promoting effect of flucocorticoids on the differentiation of human
adipocyte precursor cells cultured in a chemically defined medium. J Clin
Invest 84: 1663.
7. Katz
AJ, Tholpady A, Tholpady SS (2005) Cell surface and transcriptional
characterization of human adipose-derived adherent stromal (Hadas) cells. Stem
Cells 23: 412.
8. Miranville
A (2004) Improvement of postnatal neovascularization by human adipose
tissue-derived stem cells. Circulation 110: 349.
9. Mitchel
JB, Mcintosh K, Zvonic S, Garret S, Floyd ZE, et al. (2006) Immunophenotype of
human adipose-derived cells: Temporal changes in stromal-associated and stem
cell-associated markers. Stem Cells 24: 376.
10. Rehman
J (2004) Secretion of angiogenic and antiapoptotic factors by human adipose
stromal cells. Circulation 109: 1292-1298.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- BioMed Research Journal (ISSN:2578-8892)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Pathology and Toxicology Research
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)