1228
Views & Citations228
Likes & Shares
A 5 year old male with no past medical history presented with generalized tonic clinic seizures. Brain magnetic resonance imaging (MRI) demonstrated blurring of grey-white matter and lobar hypoplasia of right frontal lobe. Right frontal lobectomy with electrocorticography (ECoG) was performed. The final pathological diagnosis was focal cortical dysplasia (FCD) type IIa. The patient was classified as Engel class I after surgery and postoperative MRI demonstrated preservation of the right lateral ventricle.
Keywords: Cerebral cortical dysplasia, electrocorticography, epilepsy surgery, pediatric.
Abbreviations: FCD: Focal Cortical Dysplasia; MCD: Malformations of Cortical Development; AED: Anti-Epileptic Drug; ECoG: Electrocorticography; GTCS: Generalized Tonic-Clonic Seizures; EEG: Electroencephalography; MRI: Magnetic Resonance Imaging; CSF: Cerebro-Spinal Fluid; ILAE: International League against Epilepsy; FDG-PET: Fluorodeoxyglucose Positron Emission Tomography
INTRODUCTION
Epilepsy is the most common chronic neurological disease, affecting more than 65 million people worldwide [1]. Focal cortical dysplasia (FCD) is a pathological entity that belongs to a group of disorders called malformations of cortical development (MCD). FCD is a common cause of focal epilepsy in children and approximately 75% of the patients suffer from antiepileptic drug (AED) resistant epilepsy [2]. Surgery has been a treatment option for patients with certain types of epilepsy for more than 100 years [3]. FCD often requires surgical intervention to remove the affected area.
We report a patient with lesional epilepsy secondary to FCD type II who had an outstanding improvement following epilepsy surgery with electrocorticography (ECoG).
CASE
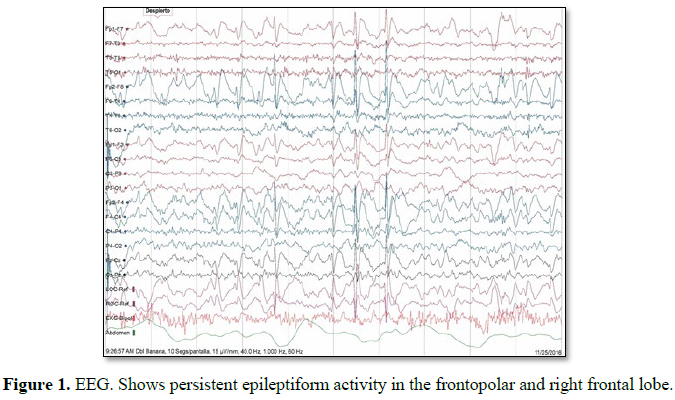
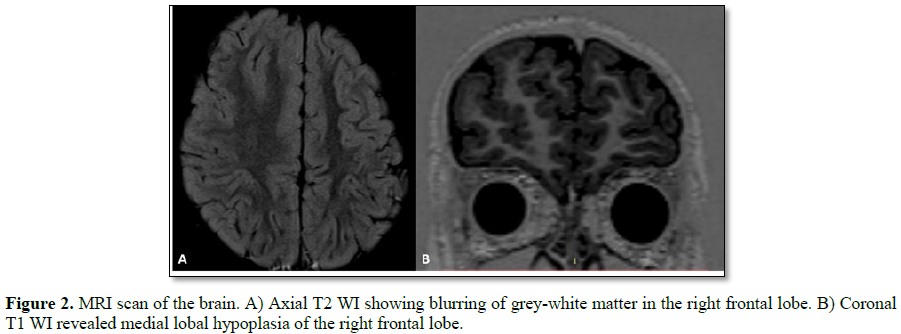
his 5 year old male, originally from Brazil, debuted in 2016 with generalized tonic-clonic seizures (GTCS) around the time of awakening. The event lasted for at least 40 s and the patient was admitted for further neurological evaluation. The patient had no past medical history. During hospital stay, the patient presented 4 more episodes around 5:00 to 6:00 in the morning and myoclonic events were observed at night; levetiracetam was initiated. Electroencephalography (EEG) revealed persistent epileptiform activity of the right frontal lobe (Figure 1) and brain magnetic resonance imaging MRI) shows sigs suggestive of FCD of the right frontal lobe (Figure 2). FCD is a common cause of medically refractory epilepsy in children [4] and surgery is a good treatment option for this pathological entity. Before surgical treatment the patient presented 6 more crises and clobazam was added to the medications. Neuropsychological testing reported developmental dyspraxia and language delay (dysarthric-dysphasic type), both associated with a developmental disorder secondary to epilepsy.
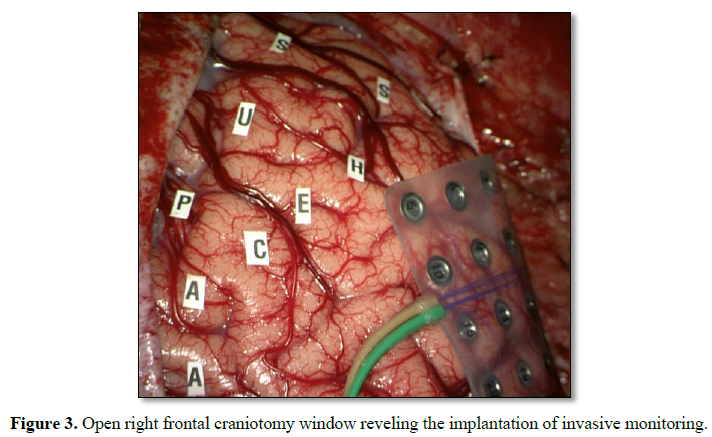
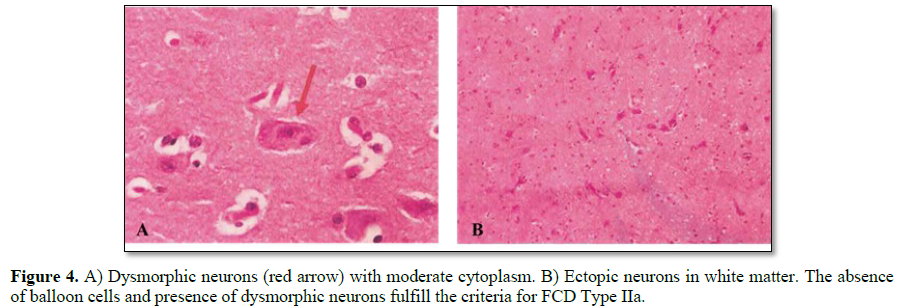
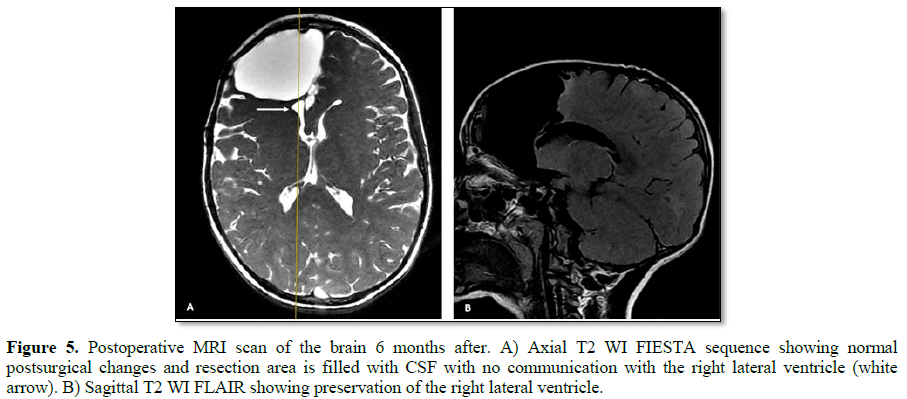
He underwent a frontal lobectomy with intraoperative ECoG and placement of subdural grids (Figure 3). During surgery the intracranial monitoring demonstrated the localization of the epileptogenic zone near to the supplementary motor area. When performing the resection of the affected frontal lobe the preservation of the right lateral ventricle was achieved. The postoperative course was uneventful. Cerebral tissue was sent to neuropathology for examination and the final pathological diagnosis of resected right frontal lobe was FCD Type IIa (Figure 4) with polymicrogyria and pachygyria. His follow-up brain MRI, taken 6 months after his surgery, indicated successful resection of the lesion, resect area is filled with cerebro-spinal fluid (CSF) and the right lateral ventricle is preserved (Figure 5). The patient remains Engel Class I and showed an improvement in language and cognition. The patient goes to a follow-up clinic, 18 months after surgery, the child understands three languages (Spanish, Portuguese and English), free of seizure, Engel 1A.
DISCUSSION
Malformation of cortical development (MCD) is a group of lesions in the brain resulting from an abnormal process in cortical development by multiple etiologies [4]. Cortical development of the brain involves a burden of processes that include neuronal stem cell proliferation, migration and differentiation. These pathological entities are a common cause of epilepsy and developmental delay [2]. FCD was first described in 1971 by Taylor et al. was they gave a detailed explanation of a specific malformation that consisted of a disorganized cortex with irregular neurons and balloon cells [5].
In 2011, the International League against Epilepsy (ILAE) Task Force for Neuropathology, proposed the currently used classification for FCD that divides it into 3 groups based on histological findings (Table 1). Our young patient presented with a Type IIa FCD, which is characterized by the presence of dysmorphic neurons, an absence of balloon cells, the impossibility to discriminate between cortical layers called cortical dyslamination and the difficulty to delineate the border between gray and white matter; other cortical layer abnormalities might be present [6]. Even though FCD can affect any lobe, Type II seems to have a predilection for the frontal lobe in contrast with Type I predilection for the temporal lobe [7].
Epilepsy is the most common clinical presentation of all types of FCD. Seizures usually begin early in childhood and the anatomical location of FCD will determine the semiology of the crisis, nevertheless, the most common type of seizure at presentation are GTCS followed by tonic seizures, focal onset aware seizures, and focal impaired awareness seizures [8,9]. MRI is an effective tool for identifying FCD, abnormalities were seen in 30% of Type I cases, 55% in Type IIa and 80% in Type IIb in a study by Leach et al. [10]. These abnormalities include cortical thickness changes, signal increase and blurriness of the gray/white matter [5]. The “transmantle sign” in MRI is an important sign for FCD Type II but is almost exclusive for subtype IIb; in fact, Type IIa is harder and not always identified on in vivo MRI [6]. Type II FCD frequently shows characteristic EEG changes such as focal rhythmic interictal epileptiform discharges that correlate with the anatomic extent of the lesion [11]. Fluorodeoxyglucose positron emission tomography (FDG-PET) is highly sensitive in detecting FCD represented by focal regional hypometabolism [8].
FCD II has been recognized as a major cause of AED resistant epilepsy [2]. Patients with drug-resistant epilepsy secondary to FCD are candidates for epilepsy surgery. In patients with FCD a complete resection is necessary, and this can be achieved with the use of intraoperative monitoring [12]. Prior surgical intervention a complete preoperative evaluation should be addressed, and it should include the following: detailed clinical history, video EEG, MR and neuropsychological testing [13]. The use of ECoG is indicated in patients with extra temporal seizures, with discordant results in the preoperative tests, and in bilateral temporal epilepsy [13]. Seizures are not typically captured on the ECoG recording. The “recruiting rhythm” an electrographic seizure on ECoG, has been reported to be a reliable marker of the epileptogenic zone in cortical dysplasia [14]. As mentioned before FCD is a common cause of intractable epilepsy in children, the use of ECoG in pediatric patients in reported in literature and approximately 35% of the patients will benefit from it [12]. Our 5 year old patient had a proper preoperative evaluation and the decision to make use of ECoG with intracranial grids was for establishing the localization of the epileptogenic zone and for determining the extent of the resection.
CONCLUSION
FCD is a pathological entity that may cause disabling seizures especially in children. Intraoperative monitoring allows neurosurgeons to have more precise resection limits with the aim to improve quality of life. We were able to preserve the lateral ventricle despite the size of the resected tissue. Our patient remains Engel class IA and he has shown great improvement in language and cognition.
ACKNOWLEDGEMENT
The authors express special thanks to Dr. Mario Arturo Alonso-Vanegas for his support.
1. Moshé S, Perucca E, Ryvlin P, Tomson T (2015) Epilepsy: New advances. Lancet 385: 884-898.
2. Pang T, Atefy R, Sheen V (2008) Malformations of cortical development. Neurologist 14: 181-191.
3. Engel J, Wiebe S, French J, Sperling M, Williamson P, et al. (2003) Practice parameter: Temporal lobe and localized neocortical resections for epilepsy. Neurology 60: 538-547.
4. Lee SK, Kim DW (2013) Focal cortical dysplasia and epilepsy surgery. J Epilepsy Res 3: 43-47.
5. Najm IM, Sarnat HB, Blümcke I (2018) The international consensus classification of focal cortical dysplasia - A critical update. Neuropathol Appl Neurobiol 44: 18-31.
6. Blümckel I, Thom M, Aronica E, Armsrong AA, Vinters HV, et al. (2011) The clinico-pathological spectrum of focal cortical dysplasias: A consensus classification proposed by and ad hoc Task Force od the ILAE diagnostic methods Comission. Epilepsia 52: 158-174.
7. Shaker T, Bernier A, Carmant L (2016) Focal cortical dysplasia in childhood epilepsy. Semin Pediatr Neurol 23: 108-119.
8. Crino PB (2015) Focal cortical dysplasia. Semin Neurol 35: 201-208.
9. Fauser S, Huppertz HJ, Bast T, Strobl K, Pantazis G, et al. (2006) Clinical characteristics in focal cortical dysplasia: a retrospective evaluation in a series of 20 patients. Brain 129: 1907-1916.
10. Maynard LM, Leach JL, Horn PS, Spaeth CG, Mangano FT, et al. (2017) Epilepsy prevalence and severity predictors in MRI-identified focal cortical dysplasia. Epilepsy Res 132: 41-49.
11. Guerrini R, Duchowny M, Jayakar P, et al. (2015) Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia 56: 1669-1686.
12. Terra VC, Thomé U, Rosset SS, Funayama SS, dos Santos AC, et al. (2014) Surgery for focal cortical dysplasia in children using intraoperative mapping. Childs Nerv Syst 30: 1839-1851.
13. Fountas K, Kapsalaki EZ (2019) Epilepsy surgery and intrinsic brain tumor surgery. 1st Edn. Switzerland: Springer.
14. Greiner HM, Horn PS, Tenney JR, Arya R, Jain SV, et al. (2016) Pre-resection intraoperative electrocorticography (ECoG) abnormalities predict seizure-onset zone and outcome in pediatric epilepsy surgery. Epilepsia 57: 582-589.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)