1242
Views & Citations242
Likes & Shares
Recent advances in peripheral nerve imaging provide a more complete
understanding of the extent of nerve injury and pathology. High-resolution
ultrasound and, more recently, MR neurography, are being increasingly used in
preoperative planning for treatment of both trauma and peripheral nerve tumors.
The addition of more advanced imaging, such as diffusion tensor imaging and
tractography has further improved preoperative planning. We review the current
techniques in imaging assessment of peripheral nerve pathology, including their
relative strengths and drawbacks, and describe the utility of a multimodality
approach to perioperative peripheral nerve imaging. This allows the surgeon to
more confidently make a decision on whether a surgical treatment is indicated
and plan for a more effective and efficient surgical approach.
INTRODUCTION
Recent
advances in imaging have provided increasingly detailed characterization of
peripheral nerve pathology. Magnetic
resonance neurography (MRN), diffusion tensor imaging (DTI) and high-resolution
ultrasound provide a more complete understanding of the nerve pathology or
injury, and thus, enable more efficient pre-operative planning [1]. This in
turn, has improved outcomes in treatment and repair of nerve pathology and
injuries [2]. In the past, detailed preoperative assessment of nerve integrity
was limited. Although the use of MRN and
nerve conduction studies have allowed for some understanding of the extent of
nerve injury or tumor involvement, it has been difficult to determine whether
surgical repair or conservative management would be more appropriate.
Furthermore, without detailed understanding of the extent or, at least,
location of nerve pathology, operative planning has been difficult. This has
resulted in long and more technically challenging procedures with risks of
complications [1].
Advanced imaging can help characterize nerve integrity in a variety of
peripheral neuropathies, most notably with tumors and traumatic injuries. With
these conditions, a multimodality approach may better characterize the extent
of nerve involvement, the relationship of nerve fibers with the tumor or site
of injury, the integrity of particular nerve fibers and whether surrounding
structures, such as vessels, are involved. With this understanding, the surgeon
can determine the most appropriate approach for repair or resection in order to
avoid injury to intact nerve fibers during surgery. Furthermore, ultrasound can
be used both preoperatively and intraoperatively to localize the nerve and more
accurately guide the operative approach.
In this
review, we describe the utility of advanced multimodality imaging to
characterize nerve pathology both preoperatively and intraoperatively.
Ultrasound
With excellent tissue
contrast, MRI is often seen as superior to ultrasound in peripheral nerve
imaging. In fact, ultrasound has many benefits over MRI and should rather be
thought of as a complementary imaging modality. Ultrasound is a real-time,
dynamic modality that allows for active interaction between the examiner and
patient. Sonographic imaging can be quickly and easily tailored to the location
and situation of the patient’s symptoms. Sonographic evaluation of a patient while
performing provocative maneuvers that reproduce symptoms may demonstrate nerve
abnormalities that cannot be detected during static imaging, such as MRI. Under
optimal conditions, ultrasound has the added benefit of higher spatial
resolution than currently available MRI scanners, which aids assessment of
smaller caliber, distal peripheral nerves. Finally, ultrasound is
well-tolerated by patients, and can be performed comfortably on patients for
whom MRI may be contraindicated or suboptimal, including those with pacemakers
or cochlear implants. Ultrasound can be performed quickly and comfortably,
obviating the need for sedation which is otherwise commonly required for MR
imaging of pediatric and claustrophobic patients.
High resolution ultrasound
is typically performed with a high frequency linear transducer (12-18 MHz) to
maximize visualization of fascicular structure. However, various factors, such
as deep location of the nerve or a larger patient body habitus, may require the
use of lower frequency transducers. Transverse images of the nerve better
delineate its fascicular structure, whereas images along the longitudinal plane
of the nerve may be useful to determine extent of injury, the degree of caliber
change that is often seen with nerve pathology, and show the relative position
of the area of nerve pathology to anatomic landmarks. Expertise in nerve
sonography is essential as suboptimal technique can mimic nerve pathology. This
is particularly the case with anisotropy, in which incorrect angle of the
transducer results in a more hypoechoic appearance of the nerve and loss of its
fibrillar pattern [3]. To verify nerve pathology, imaging must be performed by
keeping the transducer angle as close to the perpendicular axis of the target
nerve as possible, which allows optimal detection and characterization of
peripheral nerve internal architecture.
Ultrasound is readily able to
distinguish the perineural fat, which appears hyperechoic, from the hypoechoic
fascicles (Figure 1). In short axis,
the nerve fibers are round, generally similar in cross-sectional area and
separated by the more echogenic perineurium.
In long axis, the hypoechoic nerve fibers are oriented in parallel along
the long axis of the nerve, again separated by the more echogenic perineural
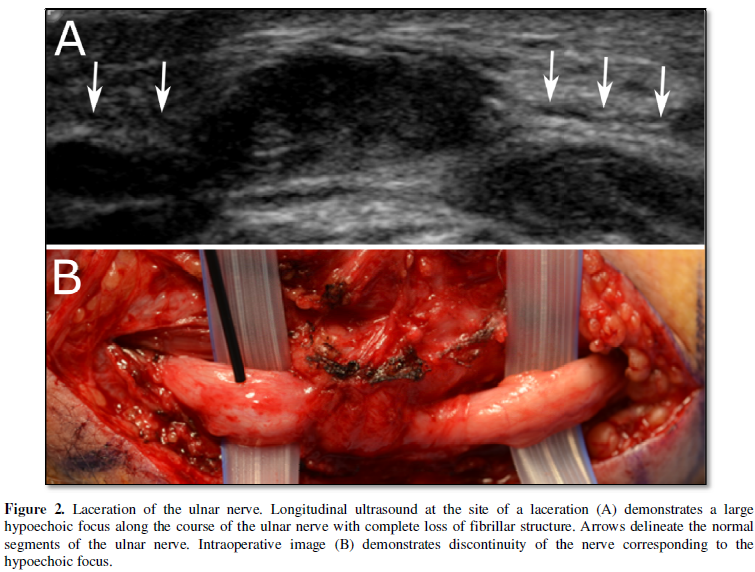
connective tissue. When injured, there is disruption of the normal fascicular
architecture of the nerve and, with increasing degree of injury, enlargement of
the nerve and discontinuity of the fibers [4] (Figure 2). In cases of compressive neuropathy, the nerve develops
an “hour-glass morphology” when seen in long axis, with the nerve swelling
proximal to the point of compression, narrowing at the point of compression,
and normal or slightly thickened nerve caliber distally.
Ultrasound has proven to be
an especially promising technique for preoperative and intraoperative planning
and localization of peripheral nerve pathology [5].Without the aid of imaging,
accessing the peripheral nerve at the site of
pathology requires a long
procedure with a wide dissection. While traditional preoperative ultrasound can
improve procedure times, differences in patient positioning between the
preoperative imaging study and surgery, can result in dramatic changes in
location of the nerve with respect to the dominant anatomic landmarks. Anecdotally, the nerve is often deeper at
surgery than the preoperative ultrasound may suggest. Therefore, ultrasound can
be used intraoperatively to better localize the region of concern, identify
potentially intact nerve fibers, and localize tumors [6] thereby helping the
peripheral nerve surgeon to determine the best surgical approach.
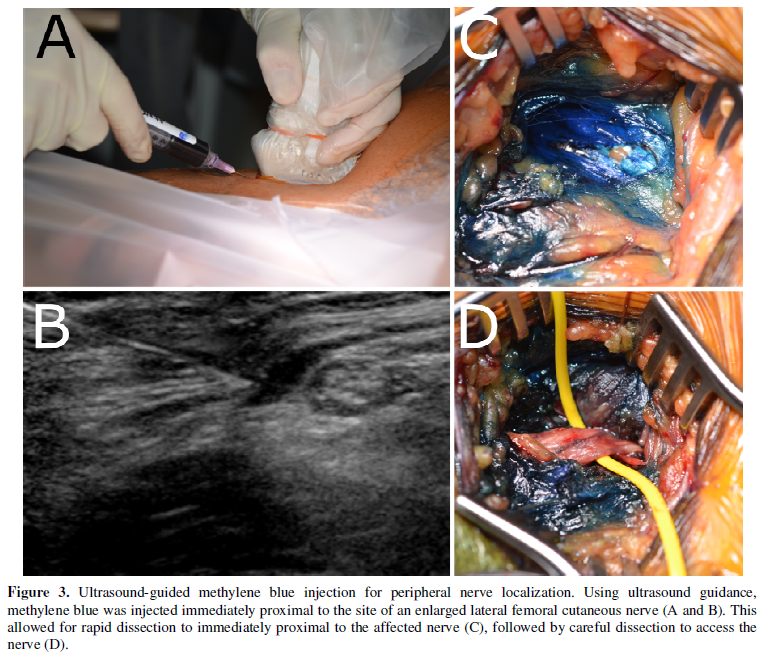
Recently, a technique using
ultrasound-guided percutaneous injection of dilute methylene blue has emerged,
providing surgeons with a better ability to both mark the site of peripheral
nerve pathology and direct surgical exploration in a more efficient manner
without the use of intraoperative imaging [7]. By injecting methylene blue near
the nerve of interest and as one is slowly withdrawing the needle, one is able
to create a surgical corridor that better directs the surgical trajectory down
to the nerve of interest. This allows for more efficient identification of the
diseased portion of the nerve (Figure 3)
and can potentially reduce operative times.
Additionally, it can provide a more accurate measure of the depth of the
nerve from the skin surface, which can also aid in more rapid surgical
exposure. Combined with the use of
electrical stimulation, it has been hypothesized that this new technique will
demonstrate clinical benefits, especially in regions of variable anatomy [5].
While outcomes and complication rates associated with this technique have yet
to be extensively studied, a number of cases reported by Osorio and colleagues with
procedures ranging from repair of impingement syndromes to tumor resection and
nerve explorations have shown promising results. Furthermore, there were no
reported associated complications, such as permanent skin discoloration, nerve
injury or hematoma [7]. While promising, further prospective studies will be
necessary to identify whether this new perioperative imaging technique will
decrease operative complications and improve surgical outcomes.
The limitations of
ultrasound include the fact that it is highly operator dependent. Expertise in
peripheral nerve sonography is required to identify the nerve of interest and
recognize nerve pathology. Factors such as the patient’s body habitus and
abnormalities in the tissue surrounding the area of concern, including
post-traumatic or postsurgical architectural distortion or scarring, can limit
the sonographic visualization of the nerve. Finally, ultrasound has limited
tissue contrast compared to MRI, which may reduce the ability of the imager to
clearly identify smaller caliber or deeper peripheral nerves. Additionally,
evaluation of masses or downstream effects of nerve pathology, such as muscle
edema or atrophy can be more difficult to identify with ultrasound.
Magnetic Resonance Neurography
MRN is useful to determine
the extent, degree and location of nerve injury. Typical MRN utilizes both
T1-weighted nonfat-suppressed and fluid-sensitive imaging. On T1-weighted
images bright signal of the perineural fat serves as a natural contrast to the
darker nerve fascicles. This enables excellent assessment of fascicular
structure and nerve caliber (Figure 4a).
Normally there is visible circumferential perineural fat that can be effaced
when the nerve is extrinsically compressed.
In cases of tumor involvement, loss of the perineural fat can indicate
impingement, encasement, and even invasion.
T1-weighted imaging is also useful in assessment of muscular fatty
atrophy as a consequence of nerve pathology, and the pattern of involved
muscles can often indicate neuropathy due to a specific peripheral nerve. Fluid-sensitive
sequences include T2-weighted or short-tau inversion recovery (STIR) sequences
and are commonly performed with fat suppression to diminish normal hyperintense
fat signal. This increases the contrast between the nerve and surrounding
tissues and facilitates better assessment of signal alterations within
individual fascicles, which can indicate neuropathy.
The fascicles in a normal
peripheral nerve are generally round and have a similar cross-sectional
area. On fluid-sensitive sequences, the
fascicles are either similar to or slightly brighter than skeletal muscle (Figure 4b). Nerve pathology often
initially presents as fascicular edema, indicated by T2 hyperintensity when
compared to other, uninvolved segments of the nerve or other regional
peripheral nerves. Occasionally, fascicular swelling can lead to
effacement of the perineurium and enlargement of the nerve. In some
neuropathies, individual fascicles may be abnormal, leading to varying sizes
and signal intensities of these fascicles. In cases of early myopathy from
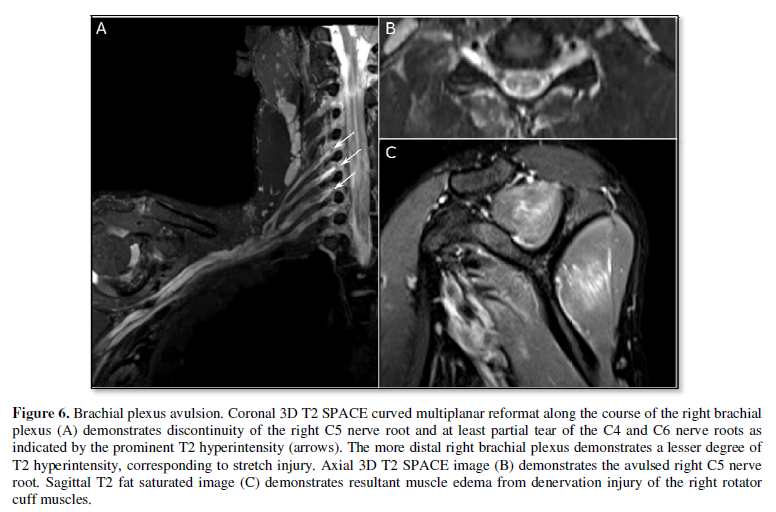
nerve injury, the affected muscle also appears hyperintense on T2-weighted
imaging (Figure 6). The utility of
intravenous gadolinium-based contrast agents is not clear, as most peripheral
nerve pathologies demonstrate T2 hyperintensity as well as enhancement. Of
course, contrast is necessary for detection and characterization of any
associated tumor. In addition, contrast
can be helpful to diagnose cases of active neuritis [8]. Furthermore, when
imaging smaller peripheral nerves that course adjacent to similarly-sized blood
vessels, intravenous contrast can help distinguish the nerve from the blood
vessels.
Unless clinically
contraindicated or not available, MRI at 3 Tesla is essential for optimal
assessment of nerves in MRN. The small size of the nerves requires the higher
signal-to-noise ratio (SNR) that 3 Tesla provides. Higher SNR serves to increase contrast and
spatial resolution, and to decrease scan times, which can become long depending
on the area of coverage needed. 3 Tesla
imaging also accentuates certain artifacts, notably metal susceptibility
artifact, which may limit assessment of nerves when there is an adjacent
implant or metallic foreign bodies. In such cases, the choice of field strength
must be determined on a case-by-case basis. The choice of coil type is another
decision that must be made prospectively and varies with the specific nerve and
suspected extent of nerve injury [9].
The selected coil should be optimal for the particular area of coverage
and is a major determinant of SNR.
Ultimately it should be tailored to the individual clinical question.
The Seddon and Sunderland
classifications of nerve injury have been correlated with changes in size,
signal and architecture of the nerve on MRI [10]. Neuropraxia, or conduction
block within the nerve without structural injury, typically is occult on
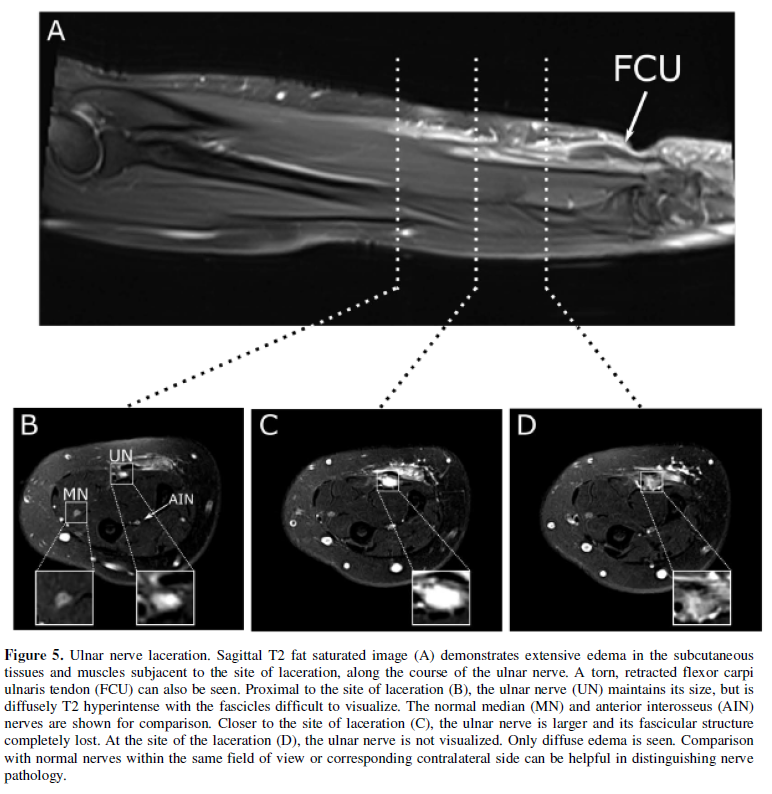
imaging. Axonotmesis, which ranges from axonal injury to perineural disruption,
can range from T2 hyperintensity with intact fascicular architecture in low
grade injuries, to nerve enlargement and fascicular disruption in higher grade
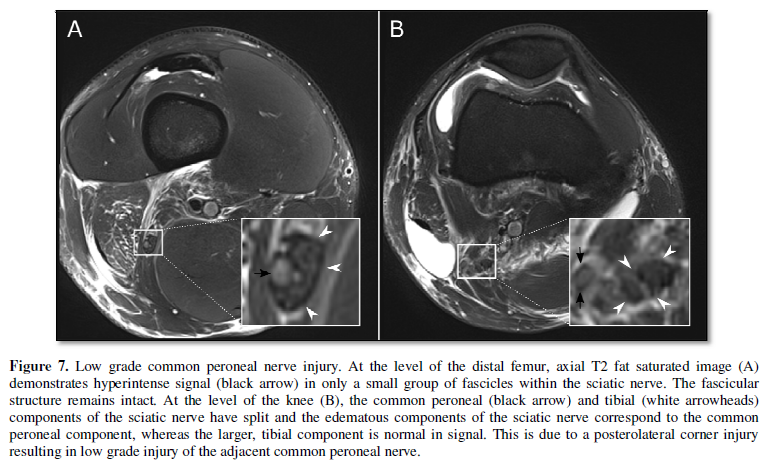
injury (Figure 5). Neurotmesis,
describes nerve disruption, which appears as discontinuity of the nerve on
imaging (Figure 6). Furthermore, injury involving specific groups
of fascicles can be identified, particularly within larger nerves, such as with
the sciatic nerve (Figure 7). When
only nerve signal is altered, without underlying structural abnormalities, the
potential for recovery without operative repair is high. As fascicular
structure is lost, the potential for spontaneous recovery without operative
repair declines [11]. Furthermore, as the nerve heals, normalization of MR
characteristics of the nerve correlates with recovery of nerve function as
determined by clinical examination and electromyography. Although traditional
MRN has been limited to more proximal, larger caliber nerves, recent use of
high-resolution 3D techniques improves visualization of the fascicular
structure within nerves, allowing for more distal nerves to be imaged and permits
reconstructions in multiple planes [12], including along the plane of the nerve
(curved multiplanar reformatting; Figure
6).
In addition to detailed
assessment of nerve pathology, MRI is essential in evaluating the surrounding
structures. Rather than direct injury to the nerve, nerve irritation can be
reactive to injury of adjacent structures (Figure
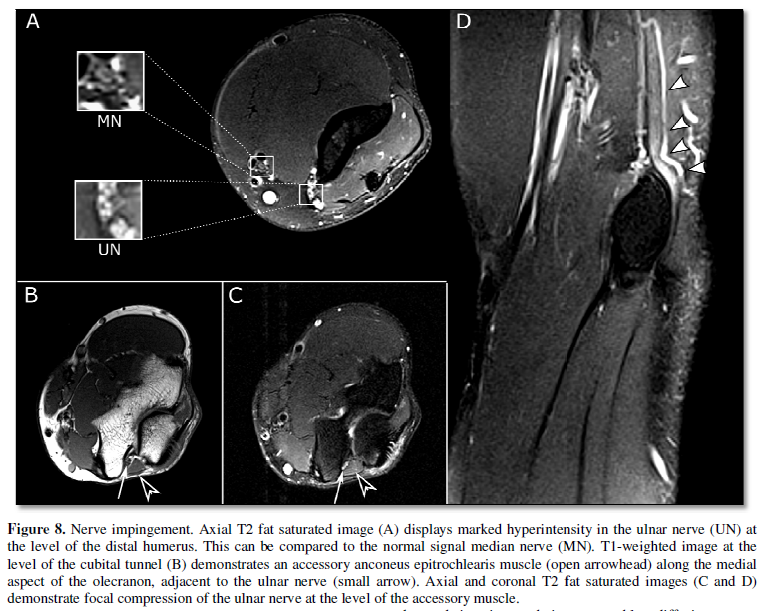
7), due to constriction by adjacent structures (Figure 8), or due to a mass. MRI can characterize these adjacent abnormalities
and establish their relationships with the nerve. This helps the surgeon to optimize
operative approach and minimize potential damage to the nerve during surgery.
Limitations of traditional
MRN lie in the fact that, despite newer 3D techniques with higher
field-strength magnets, assessment of the extent of nerve fiber injury and
tumor involvement is limited. In traditional MRN, with high-grade nerve injury,
extensive edema within nerve fibers can make it difficult to determine whether
there is complete disruption of the nerve or whether some intact fibers remain.
Occasionally, tissue architectural distortion or perineural scarring can prevent
adequate visualization of the nerve. Electromyography at the time of MRN can be
useful to detect whether some response is still present. A similar problem
arises with imaging of nerve sheath tumors, with abnormal signal from the tumor
obscuring normal nerve fibers as they pass along or through the tumor. More
advanced imaging techniques, notably, diffusion tensor imaging can be used to
address some of these issues.
Diffusion Tensor Imaging
Traditional MRN techniques
and ultrasound are anatomic techniques, and primarily evaluate the morphology
and anatomic relationships of peripheral nerves. More recently there has been emphasis on
functional assessment of the nerves.
Electrodiagnostic studies, such as nerve conduction studies and
electromyography, have been mainstays in characterizing nerve function. However, these studies can be affected by
temperature and metabolic conditions, and may not accurately determine the
location of nerve pathology [12,13]. A
recent addition to peripheral nerve imaging is the use of DTI and tractography
in characterizing anatomy and pathology of peripheral nerves. DTI is routinely
used in assessment of the integrity of white matter tracts and their
relationships to pathology in the brain. More recently, this technique has been
shown to be particularly useful in preoperative planning for peripheral nerve
tumor resection in order to balance maximal tumor resection with sparing of the
surrounding nerve fibers [14]. With higher field magnets and stronger magnetic
gradients, DTI is becoming more effective in distinguishing normal nerve fibers
from tumor during operative planning.
DTI uses the principle of
limitations in direction of water diffusivity within the constraints of a
nerve. Because of the parallel arrangement of nerve fibers, the bulk movement
of water molecules is highly limited in the transverse axis of the nerve, but
is free to move along its longitudinal axis [15]. This limitation in
directionality of water movement, or anisotropy, can be measured with MRI by
exposing the water molecules to magnetic gradients in multiple directions. The
degree of anisotropy, fractional anisotropy (FA), is high in normal nerves and
diminishes with nerve edema [16]. The degree of loss of FA in peripheral nerves
has been correlated with the extent of nerve injury and diminished potential
for spontaneous recovery of function. Furthermore, improvement of FA after
nerve injury has been correlated with improved nerve function seen both
clinically and as measured by electromyography [16]. Alterations in FA appear
earlier than changes in T2 signal and can detect subclinical neuropathy [17].
The direction of the vector
describing water movement at each location in the imaged nerve can be used to
generate tracts modeling the nerve fibers, a technique referred to as
tractography. Tractography allows for more detailed visualization and
assessment of nerve fibers by following direction of FA [18]. This can provide
detailed visualization of the site and extent of nerve injury as well as
determine whether intact fibers remain (Figure
9). In cases of peripheral nerve tumors, tractography further allows for
detailed evaluation of the relationship between nerve fibers and tumors (Figure 10 and 11). This may allow the
surgeon to modify the surgical approach in order to minimize injury to
uninvolved nerve fibers.
DTI is a technique that relies on high signal to noise ratio and should
ideally be performed on 3 Tesla scanners.
Furthermore, extensive post processing is required to remove motion and
magnetic field inhomogeneity artifacts. Thus, the technique may not be an
option in patients with metallic implants or in cases with excessive patient
motion. Tractography can be performed with various techniques and, most
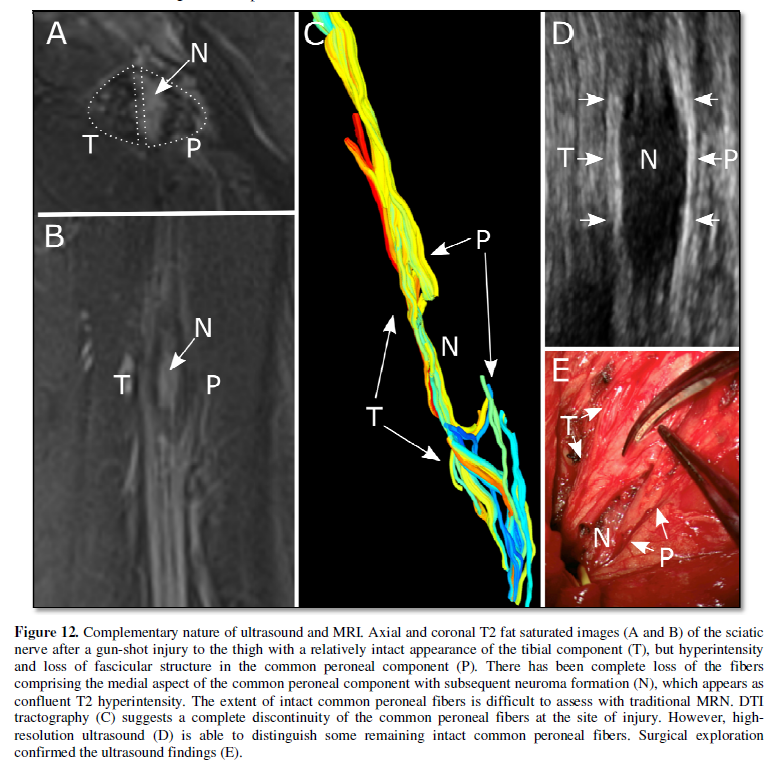
notably, requires appropriate selection of FA and angle thresholds. Improper
selection of these thresholds can overestimate or underestimate nerve integrity
(Figure 12). It should be noted the
tracts generated represent mathematical constructs detailing the direction of
bulk flow of water molecules and do not directly represent individual nerve
fibers [15]. As such, the tractograms generated can be
helpful adjunctive information to the anatomic imaging of traditional MRN and
ultrasound, and should not be used exclusively without proper understanding of
the nerve morphology and course.
A Complimentary Approach
Each of the peripheral
nerve imaging modalities has their particular strengths and drawbacks. The
choice of which modality on which to rely should be made on a case by case
basis. However, these modalities often serve as complimentary methods that,
when used in combination, can be useful to more confidently characterize nerve
pathology. For example, although MRN is excellent for assessing
characterization of peripheral nerve tumors, the relationship between
individual nerve fibers and the tumor may be difficult to delineate. The
addition of ultrasound or DTI can elucidate this relationship. Although DTI and
tractography alone are helpful tools to evaluate the integrity of damaged
nerves, the subjectivity of choosing FA and angle thresholds with which to define
intact nerve fibers may lead to overestimation or underestimation of the extent
of nerve injury. Similarly, scar tissue from prior surgery or trauma,
calcifications, metallic implants, or even a deep location may prevent adequate
visualization of the nerve in question with ultrasound, thus potentially
overestimating the degree of injury. The use of advanced imaging techniques for
preoperative planning in the setting of peripheral nerve pathology involves a
thorough understanding of the strengths and limitations of each imaging
modality and should utilize a combination of available imaging tool to ensure a
more accurate understanding of the nerve pathology.
- Chhabra A, Belzberg AJ, Rosson GD, et al. (2016) Impact
of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and
therapeutic patient management. Eur Radiol 26:1235-1244.
- Garozzo D, Basso E, Gasparotti R, et al. (2013) Brachial
Plexus Injuries in Adults: Management and Repair Strategies in our
Experience. Results from the Analysis of 428 Supraclavicular Palsies. J
Neurol Neurophys 5: 180.
- Soong J, Schafhalter‐Zoppoth I, Gray A (2005) The Importance of
Transducer Angle to Ultrasound Visibility of the Femoral Nerve. Reg Anesth
Pain Med 30: 505.
- Hollister AM, Simoncini A, Sciuk A, Jordan J (2012) High
frequency ultrasound evaluation of traumatic peripheral nerve injuries.
Neurol Res 34: 98-103.
- Gofeld M, Bristow SJ, Chiu S, Kliot M (2013)
Preoperative ultrasound-guided mapping of peripheral nerves: Laboratory
investigation. J Neurosurg 119: 709-713.
- Haldeman C, Baggott C, Hanna A (2015) Intraoperative
ultrasound-assisted peripheral nerve surgery. Neurosurg Focus 39: E4.
- Osorio J, Breshears J, Arnaout O, et al. (2015)
Ultrasound-guided percutaneous injection of methylene blue to identify
nerve pathology and guide surgery. Neurosurg Focus 39: E2.
- Thawait SK, Chaudhry V, Thawait GK, et al. (2011)
High-resolution MR neurography of diffuse peripheral nerve lesions. Am J
Neuroradiol 32:1365-1372.
- Chhabra A, Flammang A, Padua A, et al. (2014) Magnetic
resonance neurography. Technical considerations. Neuroimaging Clin N Am
24: 67-78.
- Chhabra A, Ahlawat S, Belzberg A, Andreseik G (2014)
Peripheral nerve injury grading simplified on MR neurography: As
referenced to Seddon and Sunderland classifications. Indian J Radiol
Imaging 24: 217-24.
- Thawait SK, Wang K, Subhawong TK, et al. (2012)
Peripheral nerve surgery: The role of high-resolution MR neurography. Am J
Neuroradiol 33: 203-210.
- Cho Sims G, Boothe E, Joodi R, Chhabra A (2016) 3D MR
Neurography of the Lumbosacral Plexus: Obtaining Optimal Images for
Selective Longitudinal Nerve Depiction. AJNR Am J Neuroradiol 1-5. Gooch
CL, Weimer LH (2007) The Electrodiagnosis of Neuropathy: Basic Principles
and Common Pitfalls. Neurol Clin 25: 1-28.
- Cage T, Yuh E, Hou S, et al. (2015) Visualization of
nerve fibers and their relationship to peripheral nerve tumors by
diffusion tensor imaging. Neurosurg Focus 39: E16.
- Mukherjee P, Berman JI, Chung SW, et al. (2008)
Diffusion tensor MR imaging and fiber tractography: theoretic
underpinnings. AJNR Am J Neuroradiol 29: 632-641.
- Budzik J-F, Balbi V, Verclytte S, et al. (2014)
Diffusion tensor imaging in musculoskeletal disorders. Radiographics 34:
E56-72.
- Bäumer P, Pham M, Ruetters M, et al. (2014) Peripheral
Neuropathy: Detection with Diffusion-Tensor Imaging. Radiology 273:
185-193.
- Schmidt M, Kasprian G, Amann G, et al. (2015) Diffusion
tensor tractography for the surgical management of peripheral nerve sheath
tumors. Neurosurg Focus 39: E17.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Chemotherapy Research Journal (ISSN:2642-0236)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)