2842
Views & Citations1842
Likes & Shares
There has been a
significant change in how trial sponsors engage with patients, shifting from
the traditional approach of maintaining distance from patients and treating
them as passive subjects in clinical trials to one of recognizing them as
active partners across the drug research and development continuum. In
advancing this shift, patients and their advocates are taking active roles in
discovery and pre-clinical research, in the design and execution of clinical
trials, and in leading post-market evaluation activities. Patient groups are
increasingly developing and strategically deploying assets to ensure the
perspectives of their constituents are included in clinical research, leading
to the development of treatments that meet the needs of patients while
decreasing risk and accelerating the therapy development process. As this form
of patient engagement becomes more common, evidence of its success and positive
impact on all stakeholders is mounting.
Keywords: Patient, Clinical trials, Traditional approach,
Research and development
KEY POINTS
1. Patients and the groups representing them have valuable insights and assets that can be strategically deployed to de-risk and accelerate pharmaceutical research and development.
2. The value of patient engagement in pharmaceutical research and development can be ascertained through an economic model based on expected net present value (ENPV), which is based on improved patient experience and elimination of protocol amendments in clinical trials.
INTRODUCTION
There has been a significant change in how trial sponsors engage with patients, shifting from the traditional approach of maintaining distance from patients and treating them as passive subjects in clinical trials to one of recognizing them as active partners across the drug research and development continuum [1]. As patient organizations, industry leaders, and regulatory officials work to achieve patient centricity and develop mechanisms for ensuring the patient voice is incorporated into all aspects of therapy development, evidence of the positive impact of patient engagement is mounting [2].
This paper presents the background and historical context for this evolution and evaluates the opportunities for all stakeholders, especially therapy developers, to leverage a variety of novel approaches to patient engagement for future success.
THE ADVENT OF MODERN PATIENT ADVOCACY ORGANIZATIONS: HISTORY AND CONTEXT
Patient groups, also referred to as patient advocacy organizations, disease advocacy groups, or voluntary health agencies, have been a significant part of the disease and medical research landscape for decades. Some of the earliest and most recognized models include the American Lung Association (founded in 1903) [3], the American Cancer Society (founded in 1913) [4] and the American Heart Association (founded in 1924) [5]. Through these and other national non-profit organizations, patient advocates have pursued missions of research, education, advocacy and support. Primary areas of programmatic focus for these early groups included raising philanthropic dollars to fund basic and translational research, public-facing disease awareness and education, policy advocacy and direct patient support.
The patient advocacy landscape began to shift significantly in the 1970s and 1980s. The Juvenile Diabetes Research Foundation was formed in 1970 [6] and the AIDS Coalition to Unleash Power (ACT UP) was established in 1987, with the patient community coming together to demand a more urgent government and industry response to the AIDS crisis [7].
In the 1990s, breast cancer advocates followed the example set by the AIDS community when the Komen for the Cure organization (founded in 1982) launched their Race for the Cure program in 1991 [8] in an effort to collectivize and harness the energy of a growing patient and caregiver community. The National Breast Cancer Coalition (NBCC), founded in 1991 [9], launched a campaign to bring the patient directly into the design and leadership of research programs, resulting in the creation of the Department of Defense (DoD) Breast Cancer Research Program [10].
The NBCC also pioneered the concept of engaging patient advocates directly in research programs, developing a scientific and advocacy boot camp for patients called Project LEAD [9]. This program has produced a cadre of skilled breast cancer research advocates to serve on the National Institutes of Health study sections, DoD and Patient-centered Outcome Research Institute (PCORI) [11] leadership and grant review committees and as active partners in industry sponsored drug research and development [12]. This model has been adopted by multiple patient organizations [13,14]. As a result of the continued efforts of cancer patient advocacy organizations, the Congressionally Directed Medical Research Program (CDMRP) at the DoD has expanded to cover research for multiple types of cancer, while mandating the involvement of patient advocates on grant review panels [15].
At the same time that there was an increased emphasis on policy advocacy to drive priorities of government research programs, the patient advocacy community began to develop its own capacity to identify and help fund promising research. The 1990s saw the formation of an array of patient-focused non-profit organizations that raised philanthropic dollars to fund research and research advocacy programs specifically designed to accelerate discovery and therapy development [16], including the Prostate Cancer Foundation [17] and the Michael J Fox Foundation for Parkinson’s Research [18]. These organizations saw a need and opportunity to exert influence over the research enterprise in their respective disease areas, establishing scientific and medical advisory boards and bringing research and clinical expertise in-house by hiring experts to join their increasingly professionalized staffs [19]. These trends set the stage for the dramatic shift that was to come.
PATIENT ENGAGEMENT TODAY
Many of today’s patient advocacy organizations have developed sophisticated models and programs to ensure active patient engagement across the continuum of research and therapy development [20]. Having shed the early 20th century mind-set of patients as passive beneficiaries of the research and development ecosystems, these organizations are focused on proactive and engaged efforts to participate in and drive the process of ensuring better outcomes for their constituents. The array of sophisticated, innovative initiatives undertaken by many groups today includes launching patient registries and conducting natural history studies; funding translational and early-phase clinical research programs; designing trials; developing novel trial infrastructure; leveraging venture philanthropy to drive therapy development; and conducting policy advocacy aimed at the evolving regulatory environment [21,22].
One important way in which patient organizations can contribute to drug development is by helping to fill basic research knowledge gaps about the natural course of disease progression through the conduct of natural history studies [23]. This is especially critical in areas of rare and less common diseases. Patient groups like Parent Project Muscular Dystrophy (PPMD) [24], the Cystic Fibrosis Foundation [25], Friedrich’s Ataxia Research Alliance [26], and the Platelet Disorder Support Association [27] are just a few of the pioneers in establishing sophisticated patient registries [28] (some with US Food and Drug Association [FDA] funding support) [29] that can be mined for natural history studies to establish an evidence base against which drugs can be developed.
While many patient organizations have traditionally used philanthropic support to fund basic research, these groups are increasingly directing their funding toward more translational and clinically-based research designed to prime the pump for therapy development, better identify patients who can benefit from specific therapies, and accelerate bringing new products to patients. Using targeted research grants, patient groups have funded clinical consortium efforts to develop better models and conduct early-phase clinical trials. These programs are designed to help close the gap between basic science and successful therapy development, assisting in ‘de-risking’ the work of industry [30]. For example, the Multiple Myeloma Research Foundation has established a Translational Research Network [31] aimed at establishing pre-clinical models and evaluating new drug targets.
Patient groups are moving quickly to leverage their expertise and knowledge about what their constituencies most want and need from new therapies by working with trial sponsors to select novel endpoints for trials, evolve clinical trial inclusion/exclusion criteria and develop validated outcomes measurement tools. Groups that have been active in these efforts include PPMD [32], with its pioneering efforts to define patient preferences and move away from the traditional 6-minute walk test outcome measure; the LUNGevity Foundation, with its Project Transform [33]; and Friends of Cancer Research, which worked with the American Society for Clinical Oncology to issue recommendations for broadening eligibility criteria for clinical trials in cancer [34].
These activities have evolved with patient organizations launching sophisticated clinical trials of their own, engaging multiple industry and academic partners in a robust clinical development network to accelerate therapy development. These trials have served as opportunities to address serious shortcomings in the expense and delay associated with traditional clinical development paradigms and has helped to popularize novel trial designs such as basket trials, umbrella trials, and master protocols. Examples of this work include the Pancreatic Cancer Action Network’s Precision Promise initiative [35], the Leukemia and Lymphoma Society’s Beat ALM project [36] and the National Biomarker Development Alliance’s GBM AGILE [37]. FDA leadership has publicly embraced this evolution [38].
Increasingly, patient groups have also looked to more direct engagement in drug development, leveraging a venture philanthropy model of investing directly into partnerships with industry that, if successful, could not only bring new products to patients but also yield a return on investment for the non-profit to help further advance its mission. Pioneers in this arena include the Cystic Fibrosis Foundation [39], the Juvenile Diabetes Research Foundation [40] and the Leukemia and Lymphoma Society [41].
Finally, recent years have seen a dramatic increase in the amount and type of public policy efforts undertaken by patient organizations, with specific focus on advancing patient engagement within the regulatory arena through direct engagement with the FDA, as well as through legislative initiatives to support opportunities for enhanced patient participation in drug development. These efforts were on display throughout the process of the reauthorization of the Prescription Drug User Fee Act (PDUFA) and the enactment of the 21st Century Cures legislation.
THE DRUG DEVELOPMENT ENTERPRISE RESPONDS: INDUSTRY AND REGULATORS
The medical products development field (drugs, biologics, devices) has traditionally approached patients as subjects, with drug developers and regulators generally reliant on the input of scientific and clinical experts (referred to as Key Opinion Leaders (KOLs)) as an appropriate proxy for identifying what patients need and want [42]. In this traditional model, companies generally approached patient engagement as a commercial enterprise [43], with emphasis on accruing for late-stage trials (medical affairs), designing marketing activities (patient education), and providing testimonials to assist with regulatory reviews and reimbursement (policy advocacy).
Patient organizations, reflecting the desires of their patient constituencies to be viewed as ‘KOLs in their own right’ and frustrated by the slow pace of drug development and the desire to see clinical trials and regulatory reviews that more accurately reflect the needs and desires of patients, began to advocate for transformative changes in the process of therapy development with a specific focus on changing the regulatory dynamic.
Industry, eager to respond to this trend and find ways to more systematically bring the views of their patient communities to regulators for inclusion in product reviews, began to implement shifts in corporate culture and make significant structural changes to their traditional models of patient engagement. Decisions by Sanofi and Pfizer to hire senior level patient officers [44,45] and by companies like Celgene to develop on-going cooperative programs with patient organizations (Patients’ Partners Program) [46] provide high profile examples of companies elevating patient engagement into the ranks of corporate leadership. Smaller biotech companies also experimented with developing patient partners and best practice activities to bring patient perspectives further upstream into their discovery and clinical development programs. In 2016, BIO (the trade organization representing biotech companies) published a report to assist its members and other stakeholders in developing these types of activities [42]. Research and development of medical products is undergoing a transformation to enhance patient involvement across the enterprise [47].
Recognizing the importance of enhancing and institutionalizing these activities, all stakeholders see on-going opportunity to leverage the process for reauthorizing the PDUFA. With the negotiations and commitment letters issued for PDUFA V and VI [48] and with the passage of the landmark 21st Century Cures Act, additional progress in completing the shift toward enhanced patient engagement throughout the entire drug development continuum was made. The FDA recently launched its Patient Focused Drug Development (PFDD) initiative, convening 24 public meetings organized by disease state from which a series of Voice of the Patient (VoP) reports were generated [49]. The FDA is encouraging patient organizations to host externally-led PFDD meetings [50], as the FDA turns its attention to issuing a series of guidance documents for stakeholders about incorporating the patient voice directly into product reviews [51].
OPPORTUNITIES: MAKING THE CASE FOR PATIENT ENGAGEMENT
There is emerging consensus among patient advocates, industry leaders, and regulatory officials that patient engagement early and often throughout the therapy development continuum can provide significant value to all stakeholders [52]. Specifically, the collective acceptance of the PFDD initiative is based on the view that strategic incorporation of patient perspectives (especially what they most want and need from therapy for their specific conditions) can inform clinical development strategies, improve the quality and efficiency of clinical trials and lead to more effective and meaningful therapies [53,54].
In 2014, the Clinical Trials Transformation Initiative (CTTI), a multi-stakeholder, public-private partnership between Duke University and the FDA, launched a project that recognized the significant opportunity to improve the clinical trials enterprise and enhance participation by patient groups in the work of trial sponsors. The project sought to identify barriers to meaningful engagement of patients in the development of therapeutic products, while identifying and developing consensus for best practices around the various important roles patients can play in improving the entire enterprise, from study endpoint selection that reflects outcomes of importance to patients, to recruitment and retention in clinical trials and post-marketing safety evaluation.
CTTI developed a set of recommendations [55] and tools appropriate for a regulatory environment that provide clarity around how, when, and by whom patients or patient groups should be engaged during the therapy development process, and which patients or patient groups should be engaged. The recommendations introduced practical guidance to partnership optimization for sponsors, investigators, and patient groups and were endorsed by Dr. Janet Woodcock, Director of the FDA Center for Drug Evaluation and Research, as a positive step in advancing the field of patient-focused drug development (Table 1).
Implementing a sustainable and effective approach to patient engagement and engaging patients early and often in the research and development process are both critical to delivering on the promise of less costly, more efficient and effective therapy development to more rapidly deliver better treatment options to patients [56]. As companies continue to implement these strategies, there are several areas of focus that can assist in maximizing the success of these efforts.
Most importantly, effective patient engagement requires the full commitment of everyone in an organization—from corporate leaders to researchers and developers to those involved in commercial and marketing efforts. Corporate senior leaders are increasingly embracing patient engagement as the way of the future [57].
It is vital for those within a corporate structure to engage effectively with patient organizations and individual patients; there needs to be a focus on evolving corporate culture to enable this engagement. Training should be undertaken to ensure that all within the company understand the purpose and importance of engaging with patient organizations and individual patients. Additionally, traditional legal, contracting and communication functions should be evaluated to ensure that they are designed to allow for appropriate and effective interaction with non-profit entities and individual patients.
It may be necessary to evolve corporate structures to allow for seamless integration of patient engagement across internal divisions and teams, shifting from patient interactions with only commercial and marketing teams to integration in research and development, clinical operations, and clinical innovations. It may make sense to leverage the expertise of patient alliance staff by more directly linking them to clinical development activities. It is likely that current siloed functions may need to be expanded and connected to allow for maximum success.
Finally, it should be recognized that implementing strategic patient engagement within a company (across its portfolio and pipeline) will take time and requires a long-term commitment, with the appropriate investment of resources (human capital as well as financial).
ASSESSING THE FINANCIAL VALUE OF PATIENT ENGAGEMENT
Patient advocates and regulators are convinced of the value of patient engagement efforts. Many in industry accept the assumption that incorporating appropriate patient engagement strategies will be worth the investment; however, some seek tangible data to support this hypothesis. To provide this evidence, efforts to define and quantify the return on investment have been documented in the second phase of CTTI’s patient group engagement project.
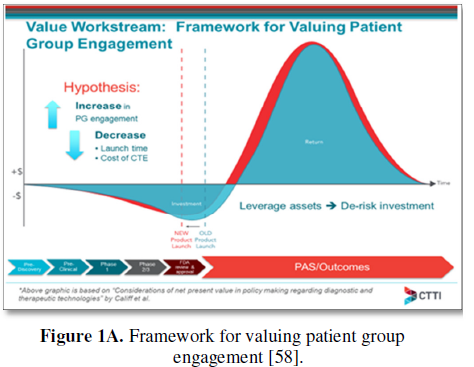
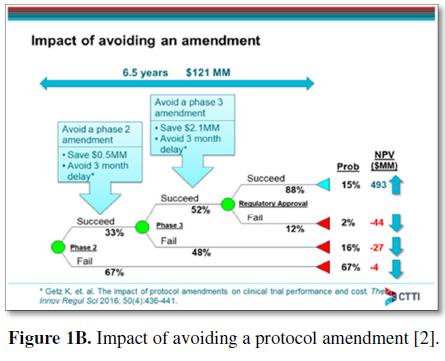
In 2016, CTTI presented a conceptual model to estimate the financial value of patient engagement based on expected net present value (ENPV), which integrates the key business drivers of cost, time, revenue, and risk into a summary metric for project strategy and portfolio decisions (Figure 1A) [2,58]. This helped the team outline what it would take to show the impact of a decrease-to-launch time and the total cost of the clinical trial enterprise as a framework for valuing patient engagement, as it has been difficult to make the fiscal value proposition. As a case example, CTTI assessed the impact of patient engagement on ENPV for a typical oncology development program entering phase 2 or phase 3. CTTI established that for a pre-phase 2 project, the cumulative impact of a patient engagement activity that avoids one protocol amendment and improves enrolment, adherence, and retention is an increase in net present value (NPV) of $62MM ($65MM for pre-phase 3) and an increase in ENPV of $35MM ($75MM for pre-phase 3). Compared with an investment of $100,000 in patient engagement, the NPV and ENPV increases can exceed the investment by 500-fold. This ENPV increase is the equivalent of accelerating a pre-phase 2 product launch by 26 years (16 years for pre-phase 3). The work determined that risk-adjusted financial models can assess the impact of patient engagement by using a combination of empirical data and subjective parameter estimates, which correlate patient engagement activities with the potential to avoid protocol amendments and/or improve enrollment, adherence, and retention (Figure 1B) [2]. The third phase of CTTI’s work is focused on developing tools to help sponsors identify high-value patient engagement activities for investment.
CONCLUSION
Patients and their advocates are taking more active roles in discovery and pre-clinical research, in the design and execution of clinical trials and in leading post-market evaluation activities. Patient groups are increasingly developing and strategically deploying assets to ensure the perspectives of their constituents are included in clinical research, leading to more rapid development of treatments that meet the needs of patients while decreasing risk and accelerating the therapy development process. Critical assessment of corporate culture and restructuring may be necessary to effectively engage patients early and often in the research and development continuum, as well as development of a structure to assess return on investment through emerging economic models.
DISCLOSURES
Selig: Nothing to report.
Patrick-Lake: Nothing to report.
1. Anderson M, McCleary KK (2015) From passengers to co-pilots: Patient roles expend. Sci Transl Med 7: 291.
2. Levitan B, Getz K, Eisenstein EL, Goldberg M, Harker M, et al. (2017) Assessing the financial value of patient engagement: A quantitative approach from CTTI’s patient groups and clinical trials project. Ther Innov Regul Sci 52: 220-229.
4. https://www.cancer.org/about-us/who-we-are/our-history.html
7. Manganiello M, Anderson M (2011) Back to basics: HIV/AIDS Advocacy as a model for catalyzing change. Available at: http://www.fastercures.org/assets/Uploads/PDF/Back2BasicsFinal.pdf
8. https://ww5.komen.org/AboutUs/OurWork.html
9. http://www.breastcancerdeadline2020.org/about-nbcc/
10. http://cdmrp.army.mil/bcrp/
11. https://www.pcori.org/about-us
12. Osuch JR, Silk K, Price C, Barlow J, Miller K, et al. (2012) Historical perspective on breast cancer activism in the Unites States: From education and support to partnership in scientific research. J Womens Health (Larchmont) 21: 355-362.
13. https://fightcolorectalcancer.org/advocacy/research-advocacy/
14. https://ww5.komen.org/GetInvolved/Participate/BecomeanAdvocate/BecomeanAdvocateinScience.html
15. http://cdmrp.army.mil/aboutus
16. https://www.healthra.org/membership/member-profiles
17. https://www.pcf.org/c/ceo-message/
18. https://www.michaeljfox.org/foundation/promise.html?navid=our-promise
19. https://www.centerwatch.com/news-online/2015/08/17/three-questions-wendy-k-d-selig-wscollaborative/
20. https://www.centerwatch.com/news-online/2015/08/17/three-questions-wendy-k-d-selig-wscollaborative/
21. http://train.fastercures.org/about/what-is-venture-philanthropy/
22. Robinson R (2016) Patients and patient organizations power rare disease therapies. PharmaVoice. Available at: http://www.pharmavoice.com/article/2016-02-rare-disease-therapies
24. http://pcornet.org/patient-powered-research-networks/pprn15-parent-project-muscular-dystrophy/
25. http://www.cff.org/Research/Researcher-Resources/Patient-Registry/
26. http://www.curefa.org/patient-registry
27. https://pdsa.org/join-the-community/registry.html
28. http://www.fastercures.org/assets/Uploads/PDF/Patient-Registries.pdf
29. FDA (2017) Awards six grants for natural history studies in rare diseases. U.S Food & Drug Administration. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm579375.htm
30. (2015) CTTI recommendations: Effective engagement with patient groups around clinical trials. Clinical Trials Transformation Initiative. Available at: https://www.ctti-clinicaltrials.org/files/pgctrecs.pdf
31. https://www.themmrf.org/research-partners/mmrf-learning-network/translational-network/
32. Peay H, Fischer R, Furlong P, Bridges JFP, Hollin I (2018) PPMD’s patient preference study about pulmonary outcomes - What we learned and why it matters. Parent Project Muscular Dystrophy. Available at: http://www.parentprojectmd.org/site/DocServer/Santhera_Community_Report_v03.pdf?docID=16886
35. https://www.pancan.org/research/precision-promise/
36. http://news.cancerconnect.com/lls-launches-groundbreaking-precision-medicine-approach-treat-alm/
37. http://nbdabiomarkers.org/gbm-agile
38. Woodcock J, LaVange LM (2017) Master protocols to study multiple therapies, multiple diseases or both. N Engl J Med 377: 62-70.
40. http://www.jdrf.org/about/t1dfund/
41. https://www.lls.org/therapy-acceleration-program
42. (2016) Key considerations in developing and integrating patient perspectives in drug development: Examination of the Duchenne case study. Parent Project Muscular Dystrophy. Available at: https://www.bio.org/sites/default/files/BIO_PPMD_whitepaper_web.pdf
43. Barron D (2017) Patient engagement: Great expectations. Eye for Pharma, Barcelona. Available at: http://social.eyeforpharma.com/commercial/patient-engagement-great-expectations
44. Schneider RF, Pankevich D (2018) Patient First” Beyond a Slogan, a Drive for Full Inclusion. Huffington Post. Available at: https://www.huffingtonpost.com/entry/patients-first-beyond-a-slogan-a-drive-for-full-inclusion_us_59c02882e4b087fdf5075781
45. Mack J (2015) The Debut of the Chief Patient Officer: Is it just a passing fad or will it transforms. Pharma Marketing News. Available at: http://www.pharma-mkting.com/news/pmnews1403-article01.pdf
46. http://www.celgene.com/partnerships/advocacy-partnerships/
48. http://www.fastercures.org/programs/r-and-d-policy/ufas/
49. Pukita V (2018) FDA’s Patient-Focused Drug Development. U.S. Food and Drug Administration. Available at: https://www.fda.gov/downloads/drugs/newsevents/ucm493616.pdf
50. U.S. Food and Drug Administration (2017) Plan for Issuance of Patient-Focused Drug Development Guidance. Available at: https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM563618.pdf
51. Kuehn CM (2018) Patient Experience Data in US Food and Drug Administration (FDA) Regulatory Decision Making: A Policy Process Perspective. Ther Innov Regul Sci 52: 661-668.
52. National Health Council (2015) Dialogue/advancing meaningful patient engagement in research, development and review of drugs. Available at: http://www.nationalhealthcouncil.org/sites/default/files/PatientEngagement-WhitePaper.pdf
53. http://phrma-docs.phrma.org/sites/default/files/pdf/patient-focused-drug-development.pdf
54. Perfetto EM, Oehrlein EM (2015) Assessing meaningful patient engagement in drug development: A definition, framework and rubric. CERSI University of Maryland.
55. Bloom D, Beetsch J, Harker M, Hersterlee S, Moreira P, et al. (2012) The rules of engagement: CTTI recommendations for successful collaborations between sponsors and patient groups around clinical trials. Ther Innov Regul Sci 52: 206-213.
56. Leonard K (2012) The blockbuster drug of the century: An engaged patient. Health Standards. Available at: http://healthstandards.com/blog/2012/08/28/drug-of-the-century/
57. Carsten B (2017) making real progress on infusing the patient voice into oncology clinical development. Medium. Availble at: https://medium.com/@bayerus/making-real-progress-on-infusing-the-patient-voice-into-oncology-clinical-development-18b3cf6f7fe4
58. Califf RM, Raisel EB, Schulman KA (2008) Considerations of net present value in policy making regarding diagnostic and therapeutic technologies. Am Heart J 156: 879-885.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Spine Diseases
- Stem Cell Research and Therapeutics (ISSN:2474-4646)