2383
Views & Citations1383
Likes & Shares
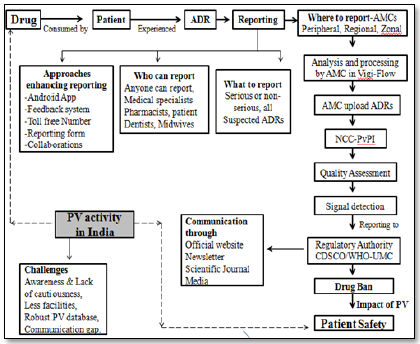
Pharmacovigilance (PV) is a specialized scientific activity that keeps constant watch on the drug and specific or uncommon Adverse Drug Reactions (ADRs) which were undetected during clinical trial. In India, the Central Drug Standard Control Organisation (CDSCO) whose headquarter is located at New Delhi regulate the PV activity. For smooth and effective working of PV a Pharmacovigilance Program of India (PvPI) was proposed and implemented by government of India in 2010. PvPI established various regional, zonal and peripheral ADR reporting centres for accurate reporting of ADR. It was cleared that any person who detected ADR can report to the nearest centre by filling Suspect ADR Reporting Form or via telephone and email etc. The reported ADRs are collected and processed at the respective centres in Vigi-flow software. The associate at these centres detect signal, reported to CDSCO and World Health Organisation (WHO) for the further regulatory action. CDSCO-WHO individually or in collaboration communicates their decision via newsletter, media, journal or official website in favour of public health.
Keywords: Pharmacovigilance, Adverse drug reaction, Pharmacovigilance Program of India, Vigi-flow, Central Drug Standard Control Organisation
Abbreviations: ADR: Adverse Drug Reactions; PvPI: Pharmacovigilance Program of India; CDSCO: Central Drug Standard Control Organisation; WHO: World Health Organisation; All AIIMS: India Institutes of Medical Sciences; AMCs: ADR Monitoring Centres; PV: Pharmacovigilance; ASU: Ayurveda, Siddha, Unani; UMC: Upsala Monitoring Centres; HCPs: Health Care Professionals; NCC: National Coordinating Centre
Clinical trial (Phase I-III) safety data of drug decides the launching of drug in the market, whereas phase IV study reveals post marketing surveillance. Clinical studies on limited population detect only common Adverse Drug Reaction (ADR) but, the reaction which takes time to develop remains undetected. Pharmacovigilance (PV) is a scientific activity which keeps constant watch on drugs safety throughout the life cycle and deals with the understanding of ADR associated with it. The noxious and unintended reactions occurring at normal therapeutic dose are named as ADRs [1]. While, the untoward events occurred during drug therapy having no relation with its use are called “adverse event” [2].
In 1961, thalidomide tragedy acted as a trigger to enlighten the drug safety issue, this issue was globalized and hence World Health Organization (WHO) in 1968 established international drug monitoring program [3]. It was found that almost all the drugs are associated with the beneficial as well as harmful reaction. The French scientist coined the term PV which help to minimize ADR along with the assessment of risk benefit ratio [4,5].
In India clinical trial was started in 1996 and within two year of this, India joined ADR monitoring program with the initiation of the PV activity. This attempt was not enough, hence; on 14 July 2010 Government of India started the PV Program for India (PvPI) [6]. As part of this PvPI; All India Institutes of Medical Sciences (AIIMS), New Delhi selected as National Coordinating Centre (NCC) to safe-guard public health by validating the safety of products. In addition for smooth and efficient functioning of the program various eligible medical colleges were selected as ADR Monitoring Centres (AMC) [7]. Till January 2017, 250 AMCs (government and non-government) have been established under PvPI. ADR data for Ayurveda, Siddha, Unani (ASU) medicines was essential and leads to the establishment of PV for AYUSH drugs as per WHO guidelines [8].
ADR REPORTING CRITERIA [9-14]
FACILITIES FOR INCREASING PV ACTIVITY
Number of efforts has been taken by NCC-PvPI for the enhancement of the reporting of ADR. India is now a well-connected nation in terms of telecommunication and internet. Considering this connectivity PvPI on 11 October, 2013, started toll free helpline number (1800 180 3024) and on 15 May, 2015 launched Android Application for faster reporting of ADR [15,16].
To increase interest of consumer and Health Care Professionals (HCPs) a feedback letter or form facility was started [9]. All the universities incorporated PV as curricular subject and some private institution have started providing professional courses and training on PV [17].
India is a multi-linguistic nation, for the better understanding of consumer, reporting forms are prepared in vernacular languages which are available 24 × 7 on official website (pvpi.compat@gmail.com). In India it is mandatory for the Marketing Authorization Holders (MAH) to submit PSUR (Periodic safety update reports) to CDSCO twice a year for 2 consecutive years; this attempt helps to collect safety data of on-going marketed product regularly [18].
To get the professional knowledge, technical support, and enough ADR data, collaborations with the various governments, non-government organisation, and WHO-UMC Upsala Monitoring Centre was increased and strengthen [10,18].
ADR PROCESSING
All the ADR reports from various sources are collected at the nearest AMC’s. PV staff at AMC analyses and prioritise the collected report and perform provisional causality assessment. The assessed ADR forms are then directed towards the coordinating centres to conduct final causality evaluation. Thus evaluated reports feed into the PV database; aggregate report of collected ADRs forms gets prepared and sends it to WHO-UMC. Finally, the ADR data is transferred through Vigi-Flow database to the UMC database. Expert at UMC analyses the submitted data and checks the drug-ADR relationship called as a signal. This signal acts as alarm or message which is then communicated with NCC-PvPI via CDSCO to restrict further use of suspected drug in India. A separate quality review panel exists for the maintenance of processed ADR quality [17,19,20].
COMMUNICATION OF ADR
NCC communicates important finding and knowledge of ADR with stakeholders and public via following mean [9,18]:
1. Newsletters available in secured PDF format issued by PvPI every year to guide HCPs.
2. Scientific journals published by NCC.
3. Media (Advertisement) serves as communication medium for the safe and rational use of drug.
4. Official websites like www.cdsco.nic.in (CDSCO) and www.ipc.gov.in (NCC) acts as source of information about list of AMCs, reporting procedure, etc.
CHALLENGES TO PV
The peoples and HCPs are less cautious about health and under reporting was the basic concern in Indian PV system. Its fact, many a time patient may not reach to the physician or HCPs for care and ADR remains undetected leads to reporting failure. Sometime HCPs are less enthusiastic about the reporting of ADR. Absence of robust PV system or software at PvPI to process ADRs and lack of interest of HCPs in reporting are the serious issues. There is less involvement of the pharmacy store in-charge in ADR reporting because of absence of power and legal binding.
Currently Indian PV system is in developing stage and the initiatives taken like toll free number, android app, training, feedback forms etc. have enhanced public or HCPs interest in reporting of ADR.
CONCLUSION
In India PV system has increased awareness among the people regarding health, rational use of drug and ADR reporting. The issue of under reporting is resolving due to initiatives taken by NCC-PvPI. This may not be enough at all, government need to focus on the active involvement of HCPs, pharmacist must get right to practice as like in western countries, because every time patient may not reach to physician. A robust PV system needs to be built along with more number of linked AMCs. Separate PV cell at every multi-speciality hospital and robust PV system will surely enhance PV activity.
CONFLICT OF INTEREST
None
FUNDING SOURCE
None
1. World Health Organization (1984) Collaborating Centre for International Drug Monitoring. Geneva, WHO publication DEM/NC/84.153 (E).
2. World Health Organization (2002) Collaborating Centre for International Drug Monitoring. The importance of pharmacovigilance. Safety monitoring of medicinal products. Geneva: World Health Organization.
3. Kim JH, Scialli AR (2011) Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol Sci 122: 1-6.
4. Bégaud B, Chaslerie A, Haramburu F (1994) Organization and results of drug vigilance in France. Rev Epidemiol Sante Publique 42: 416-423.
5. Routledge (1998) 150 years of pharmacovigilance. Lancet 351: 1200-1201.
6. Kumar S, Baldi A (2013). Pharmacovigilance in India: Perspectives and prospects. J Drug Deliv Ther 3: 237-246.
7. Protocol for National Pharmacovigilance Program (2004) CDSCO, Ministry of Health and Family Welfare, Government of India.
8. Protocol National Pharmacovigilance Program for Ayurveda, Siddha and Unani (ASU) Drugs. Sponsored by Department of AYUSH, Ministry of Health & Family Welfare, Government of India, New Delhi.
9. http://www.ipc.gov.in/PvPI/adr.html
10. http://www.ipc.gov.in/PvPI/oi.html
11. Mann RD, Andrews EB (2002) Spontaneous reports are very useful. Pharmacovigilance. Eds. John Wiley & Sons Ltd., Chichester.
12. Srivastava P, Kumar P, Sharma A, Upadhyay Y (2011) A review on pharmacovigilance importance and current regulations. Pharmacol Online 2: 1417-1426.
13. Kalaiselvan V, Prasad T, Bisht A, Singh S, Singh GN (2014) Adverse drug reactions reporting culture in Pharmacovigilance Programme of India. Indian J Med Res 140: 563-564.
14. van Grootheest K, de Graaf L, de Jong‑van den Berg LT (2003) Consumer adverse drug reaction reporting: A new step in pharmacovigilance? Drug Saf 26: 211-217.
15. Kalaiselvan V, Mishra P, Singh GN (2014). Helpline facility to assist reporting of adverse drug reactions in India. WHO South-East Asia J Public Health 3: 194.
16. Kuchya S, Kalaiselvan V, Kaur I, Singh GN (2016) Mobile application an approach to enhance easy adverse drug reactions reporting in India. Health Technol 6: 157-158.
17. Kalaiselvan V, Sharma S, Singh GN (2014) Adverse reactions to contrast media: An analysis of spontaneous reports in the database of the pharmacovigilance program of India. Drug Saf 37: 703-710.
18. Kalaiselvan V, Thota V, Singh GN (2016) Pharmacovigilance programme of India: Recent developments and future perspectives. Indian J Pharmacol 48: 24-28.
19. Safety of Medicines (2002) A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. Geneva, World Health Organization. WHO/EDM/QSM/2002.2.
20. Palanisamy S, Kottur SG, Kumaran A and Rajasekaran A (2013) A study on assessment of knowledge about adverse drug reactions. Der Pharmacia Lett 5: 41-52.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Alcoholism Clinical Research
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)