830
Views & Citations10
Likes & Shares
Objective: Bone resorption is the cornerstone in bone
remodeling affecting the rate of orthodontic tooth movement. Different
cytokines have a direct effect on osteoclastogenesis. The aim of this study is
to evaluate the effect of Low Level Laser Therapy (LLLT) RANKL release during orthodontic
tooth movement.
Materials and methods: 20 patients requiring orthodontic
therapeutic extraction of the maxillary first premolars were randomly selected.
A randomized controlled trial was preformed; Laser group (LG) was assigned
using 940 nm diode laser irradiations (100 mW, 2.5 J, 3.9 J/cm2) at
days 1, 3, 8 and 15. Canine retraction was done using nickel-titanium
closed-coil spring applying a force of 150 g/side. Gingival crevicular fluid
(GCF) samples were collected from distal surface of the canines on both sides,
1 day before the intervention treatment (T0), day 3 after the intervention
(T1), day 15 (T2). RANKL concentration levels were assessed using Enzyme linked
immunosorbent assay.
Results: There was statistically significant increase
in RANKL concentration levels in the Laser Group from T0 to T1, there was no
statistically significant difference in concentration level from T1 to T2, but
there was a statistically significant difference in RANKL concentration levels
between T0 and T2 in both groups.
Conclusion: Low Level laser therapy can increase the
RANKL release during orthodontic tooth movement.
Keywords: Laser therapy, Cytokines, Orthodontic tooth,
Antiretroviral therapy
INTRODUCTION
The receptor
activators of nuclear factor kappa B ligand (RANKL) molecule exerts
counterbalancing regulatory effects on osteoclastogenesis, including osteoclast
differentiation, activation and survival and are as a result critical for
initiation and maintenance of orthodontic tooth movement. Osteoclast
differentiation and function appear to be regulated by a counterbalancing
system, which has been referred to as the RANKL/RANK/OPG regulatory axis. An
increased RANKL/OPG ratio will favor osteoclast formation and activation, so
bone resorption will occur [3-6].
Low level laser
therapy (LLLT) has yielded important outcomes in orthodontics, with positive
effects on bone remodeling and acceleration of new vascularization and
acceleration of tissue healing and repair. Youssef et al. [7] evaluated the
effect of the low-level (GaAlAs) diode laser (809 nm, 100 mW) on the canine
retraction their findings suggested that low-level laser therapy can highly
accelerate tooth movement during orthodontic treatment and can also effectively
reduce pain level. Altan et al. [8] studied the effects of 820 nm diode laser
on osteoclastic and osteoblastic cell proliferation-activity and RANKL/OPG
release during orthodontic tooth movement they concluded that low-level laser
irradiation accelerates the bone remodeling process by stimulating osteoblastic
and osteoclastic cell proliferation and function during orthodontic tooth
movement. Fujita et al. [9] study was designed to examine the effects
of low-
The aim of this study is to evaluate the
effect of LLLT applications during orthodontic tooth movement on the release of
RANKL.
MATERIALS AND
METHODS
This study was reviewed and approved for
scientific validity and methodology by the committee of postgraduate studies
and research, Faculty of Dentistry, Suez Canal University. Subjects were
selected from the orthodontic outpatient clinic, faculty of dentistry, Suez
Canal University and Misr International University. Adult patients with full
permanent dentition seeking orthodontic treatment were free from any medical
condition that may interfere with the orthodontic treatment. Patients having
good oral hygiene and free from periodontal problems. Patients who did not have
previous orthodontic treatment. Patients diagnosed for Class II division 1
malocclusion with increased over jet or patients with bimaxillary dentoalveolar
protrusion malocclusion; that will require therapeutic extraction of upper
first and second premolars and retraction of the anterior segment. Potential
participants were informed of the study rationale and design. In addition, they
were provided with a written consent for approval to participate in this study.
The full sample following the approval of the informed consent comprised of 20
patients (8 males, 12 females) age ranging from 18 years to 29 years.
Sample size calculation was based upon the
results of Nishijima et al. [11] using RANKL level as the primary outcome; the
effect size was large (1.02). Using alpha (α) level of (5%) and Beta (β) level
of (20%), i.e., power=80%; the minimum estimated sample size was 10 subjects.
Sample size calculation was performed using IBM® SPSS® SamplePower®
Release 3.0.1; accordingly, the sample size was determined to be 20 subjects.
For every patient a fixed upper and lower
orthodontic appliance (OrmcoTM mini 2000 Roth slot 0.22, USA) were used. After
leveling and alignment of the upper arch, mini-screws (MCT BIOTM, QmedicalÒ, South Korea) were placed
bilaterally between the upper first molar and second premolar below the
mucogingival junction in the attached mucosa.
After leveling and alignment and reaching a
heavy arch wire (0.016 × 0.022 stainless steel wire) and for each patient the
intervention technique was assigned to a quadrant: Low Level Laser Therapy
(LLLT) was assigned to the other side of the maxillary arch also at the canine
region.
Gingival crevicular fluid (GCF) samples were collected using PERIOPAPERÒ strips (Harco, Tustin, Calif). The site to be sampled was isolated with
cotton rolls and plaque was gently removed with cotton pellets. Sites were then
washed with water and air dried prior to sampling. The filter paper strip was
inserted 1-2 mm into the gingival sulcus until mild resistance is felt. It was
left in position for 30 s whilst GCF is absorbed into it. Care was taken to
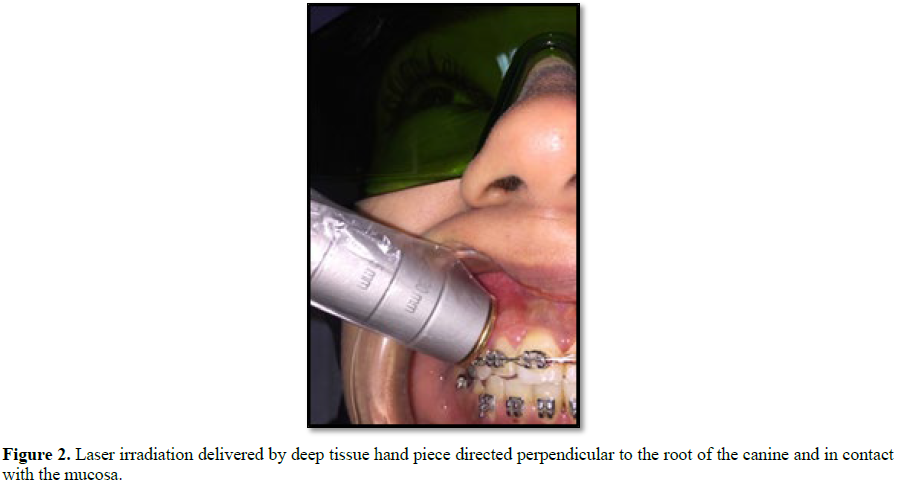
avoid damage to the soft gingival tissues (Figure 2) [12].
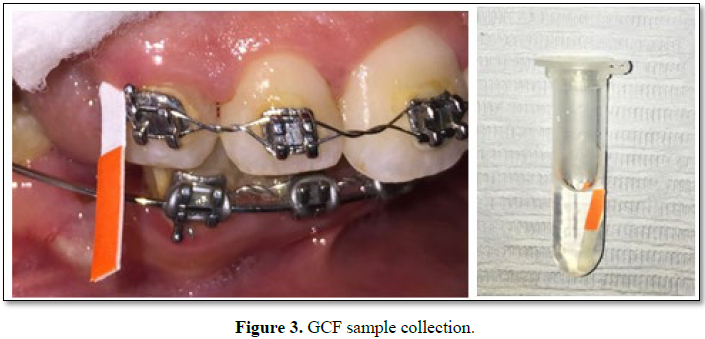
Samples were collected from the distobuccal sites of the examined canines
from both groups as follows (Figure 3 and Table 1):
The detection of RANKL was done by ELISA technique using Fine Test kit
cat number (EH0313). This technique was based on sandwich enzyme-linked
immune-sorbent assay technology. The test samples; gingival percravicular
miniprep were embedded in phosphate buffer saline (PBS) with pH 7.5 soon after
collecting and aliquot and stored at -80°C for long term with avoidance of
multiple freeze-thaw cycles. The reagents allowed warming for at least 30 min
at room temperature (37°C); the samples were diluted and mixed completely and
evenly. The standard was settled, test sample and control (zero) wells on the
pre-coated plate respectively and then their positions were recorded. The
standard was used in different gradient concentrations according to the
manufacture instructions. The prepared standards were added into the
appropriate wells at a volume of 0.1 ml; similarly, samples were added also
into test sample wells. The plate was sealed with a cover and incubated at 37°C
for 90 min. The cover was removed and the plate content was discarded, the
plate was clapped on the absorbent filter papers or other absorbent material.
Do NOT let the wells completely dry at any time. The 0.1 ml of Biotin-detection
antibody working solution was added into the above wells (standard, test sample
and zero wells). The solution was added at the bottom of each well without
touching the side wall. The plate was sealed with a cover and incubated at 37°C
for 60 min. The cover was removed and the plate washed 3 times with Wash Buffer
A 0.1 ml of SABC working solution was added into each well, the plate covered
and incubated at 37°C for 30 min. The plate washed 5 times with Wash buffer and
each time we let the wash buffer stay in the wells for 1-2 min. A 90 μl of TMB
substrate was added into each well; the plate was coved and incubated at 37°C
in dark within 15-30 min. A 50 μl of Stop solution was added into each well and
mixed thoroughly. The color changed into yellow immediately. The O.D.
absorbance was read at 450 nm in a microplate reader immediately after the stop
solution has been added. The concentration of the measured parameter was
calculated using the following equation:
The relative O.D.450 = The O.D.450 of each well – The O.D.450 of Zero
well
The standard curve was plotted as the relative O.D.450 of each standard
solution (Y) vs. the respective concentration of the standard solution (X). The
concentration of the measured parameter in the samples was interpolated from
the standard curve; the Curve was plotted using specific professional software;
finally, the samples calculated results were multiplied by the dilution factor
to the concentrations from interpolation to obtain the concentration before
dilution.
Numerical data were explored for normality by checking the distribution
of data and using tests of normality (Kolmogorov-Smirnov and Shapiro-Wilk
tests). Data showed normal (parametric) distribution. Data were presented as
mean, standard deviation (SD) and 95% Confidence Interval (95% CI) for the mean
values.
Two-way repeated measures Analysis of Variance (ANOVA) was used to study
the effect of treatment, time and their interaction on mean sRANKL
concentration. Bonferroni’s post-hoc test was used for pair-wise comparisons
when ANOVA test is significant. The significance level was set at P ≤ 0.05.
Statistical analysis was performed with IBM®[1] SPSS®
Statistics Version 20 for Windows.
RESULTS
Demographic data
The present study was conducted on 20 subjects; 8 males (40%) and 12
females (60%). The mean and (standard deviation) values for age were 23.4 (3.5)
years with a minimum of 18 and a maximum of 29 years old.
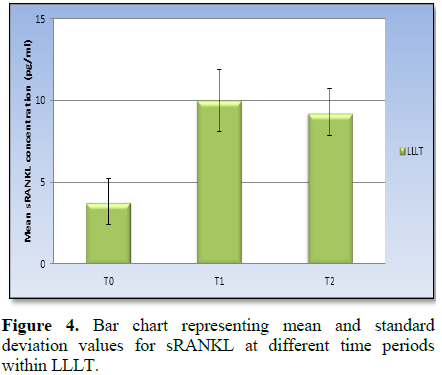
sRANKL concentration
With LLLT treatment; there was a statistically significant change in mean
sRANKL concentration by time (P-value <0.001, Effect size=0.880). Pair-wise
comparisons between the follow up times revealed that there was a statistically
significant increase in mean sRANKL concentration from T0 to T1 followed by
non-statistically significant decrease in sRANKL concentration from T1 to T2.
The mean sRANKL level at T2 showed statistically significantly higher value
compared to T0 concentration (Figure 4 and Table 2).
DISCUSSION
Several techniques are currently used to accelerate orthodontic tooth
movement. In this study we investigated the effect of the release of RANKL
during orthodontic tooth movement. We distinguished noninvasive approach: Low
Level Laser Therapy (LLLT).
The sample size used in this present study was larger than the sample
size in Nishijima et al. [11] study investigating the levels of RANKL and OPG
in GCF for 10 subjects undergoing orthodontic tooth movement. Again it was
larger than the sample size used in Barbieri et al. [13] study where Levels of
RANK, OPG, OPN and TGF-ß1 were also analyzed in 10 volunteers undergoing
orthodontic treatment. However, our sample size in the present study matched
the same size as in Grant et al. [14] study investigated changes in cytokines
and biomarkers of bone and tissue metabolism within gingival crevicular fluid
(GCF) from patients undergoing orthodontic treatment.
The effect of LLLT on the rate of OTM was previously investigated in
previous studies. Seifi et al. [17], Gama et al. [18] and Marquezan et al. [19]
performed animal studies and they all shared similar results and stated that
there was no statistically significant difference between the irradiated group
and non-irradiated groups on the rate of OTM. In contrast to their results
Altan et al. [8], Suzuki et al. [10], Yoshida et al. [20], Yamaguchi et al.
[21] and Cossetin et al. [22] performed animal studies and stated that LLLT can
improve the rate of OTM through high expression of RANKL release stimulating
alveolar bone remodeling. Youssef et al. [7], Dosh-Mehta et al. [16], Genc et
al. [23], Caccianiga et al. [24], Qamruddin et al. [25] and Arumughan et al.
[26] in their clinical studies on patients stated that LLLT had a significant
effect on accelerating the rate OTM.
In the current study near infrared 940 nm InGaAsP Diode laser was used,
Qamruddin et al. [25] used the same wavelength 940 nm in their clinical study
investigating the effect of LLLT on rate of OTM during canine retraction in
Class II div 1 patients.
Baxter and Diamantopoulos [27] stated that laser wave length and energy
density are the most important factors determining the tissue response. Mester
et al. [28] stated that energy density in the 0.5-4 J/cm2 is the
most effective range in start of a photobiological tissue reaction. According
to these findings the energy density used in the study was 3.9 J/cm2;
that was calculated from the given parameters:
Energy Density = Energy (J)/Area (cm2)
In the current study GCF samples were collected from the distobuucal
sites using periopaper strips and the markers for RANKL were evaluated using
ELISA test and the concentration of RANKL was expressed in pictogram/millimeter
(pg/ml), in the study done by Bariebri et al. [13] RANKL was measured through
GCF samples collected using periopaper strips at the mesiobuccal (tension side)
and distobuccal (compression side) sites. The amount of each biomarker was
determined in picograms (pg). Another study using a similar technique for
sampling was Rody et al. [29] investigating differences in the GCF composition
between adults and adolescents undergoing orthodontic treatment.
The results of the current study stated that after applying LLLT there
was a significant increase of sRANKL concentration levels, there was a significant
increase of from T0 (before laser application) to T1 (day 3 of laser
application), the mean sRANKL level at T2 (day 15 of LASER application) showed
statistically significantly higher value compared to T0 concentration;
P-value<0.001, Effect size=0.880. These results comply with results stated
by Fujita et al. [9] where they investigated the concentration levels of RANKL
at days 2, and 3 after low laser laser irradiation during orthodontic
treatment; they stated that the positive immunoreactions to the primary
antibodies of RANKL and RANK were significantly increased in the irradiation
group on day 2 and 3, compared with the non-irradiation group, they concluded
that These findings suggest that low-energy laser irradiation stimulates the
velocity of tooth movement via induction of RANK and RANKL.
Altan et al. [8] investigated the effect of different laser irradiations
parameters in comparison to control group on the rate of OTM, they found that
the group irradiated with lower energy density findings showed that RANKL
immune-reactivity was stronger than in the other groups. Milligan et al. [30]
designed a study to evaluate the effect of two different wattage parameters of
LLLT on orthodontically moved molars, exhibited differences in the amount of
tooth movement and molecular and histological changes in the adjacent
periodontal areas. Their findings suggested that regardless of wattage used the
laser irradiated groups exhibited higher and significant increase level of
RANKL concentration levels when compared to control non-irradiated groups.
Similar results were also stated by Suzuki et al. [10]; in their study they
investigated the effect of LLLT on rate of OTM and concentration levels of
RANKL at days 3, 6, 9 and 21. The immunohistochemistry analysis showed
increasing number of TRAP-positive osteoclasts and the RANKL expression at the
compression side, during all examination times. Which complies with this
current study results that showed significant increase of RANKL concentration
at day 3 (T1) and day 15 (T2) when compared to T0; also there was no
significant change of RANKL concentration between day 3 (T1) and day 15 (T2),
implying that the used frequency of irradiations (day 1, day 3, day 5, day 8,
day 15) maintained high level of RANKL and we can postulate that with repeating
the fore-mentioned protocol we can maintain RANKL concentration throughout the
canine retraction procedure. Another study stating the positive effect of LLLT
on RANKL concentration was the study of Aihara et al. [31] that evaluated
preosteoclast-like cells to measure the amount of RANK after radiation in
vitro. Immunohistological staining and RT-PCR expressed higher levels of RANK
and RANKL in the laser therapy group as compared to the control group. Kim et
al. [32] also evaluated the amount of RANK/RANKL using 2 immunohistochemistry
analyses. They realized that RANKL levels were significantly in higher
concentration levels in the laser group from the beginning to the end of the
study.
In contrast to the results of Dominguez et al. [33], where they
investigated the effect of LLLT on accelerating rate of OTM, pain and RANKL
concentration in GCF. Their results showed that although there was improvement
and increase in RANKL concentration levels, in the rate OTM and pain
perception, yet the change was not significantly different from the control
group. Furthermore, three studies showed that LLLT did not statistically
accelerate orthodontic tooth movement; these are studies done by Marquezan et
al. [19], Limpanichkul et al. [34] and Kansal et al. [35]. The literature
reports that energy density is the most important laser parameter to be
considered as it determines the amount of energy received by the tissues per
area. An acceptable energy density is between 0.5 and 12 J/cm2;
however, the ideal energy density seems concentrated between 1 and 6 J/cm2.
Marquezan et al. [19] used in his study energy density of 6000 J/cm2,
in Limpanichkul et al. [34] study the energy density used was 25 J/cm2
while in the current study the energy density used was 3.9 J/cm2.
The higher energy density may be the reason they showed no difference between
the experimental low level-intensity laser therapy subjects and the controls in
both studies. In the study of Kansal et al. [35] used outcome power was 12 mW
that would unlikely produce a direct photochemical or biostimulatory effect. In
the present study the output power used was 100 mW.
Aligning with the results found in this study, the review article
published by Yassaei et al. [36] stated that based on different researches, it
may be concluded that low level laser therapy may increase the rate of tooth
movement during orthodontic treatment through the mechanism of increasing
levels of RANKL in PDL which leads to increased osteoclastogenesis.
CONCLUSION
Low level laser
therapy facilitated orthodontics applications will increase the RANKL release
during orthodontic tooth movement. As RANKL is a key molecular factor in
osteoclastogenesis, we can assume that LLLT and CFO can accelerate the rate
OTM.
1. Xie R, Kuijpers-Jagtman A, Maltha
J (2008) Osteoclast differentiation during experimental tooth movement by a
short-term force application: An immunohistochemical study in rats. Acta
Odontologica Scandinavica 66: 314-320.
2. Shetty S, Kumar M, Smith PL (2011)
Role of cytokines in orthodontic tooth movement. J Dent Sci Res 2: 132-141.
3. Brooks P, Nliforoushan D, Manoison
M, Simmons C, Gong S (2009) Molecular markers of early orthodontic tooth
movement. Angle Orthod 79: 1108-1113.
4. Boyle WJ, Simonet WS, Lacey DL
(2003) Osteoclast differentiation and activation. Nature 423: 337-342.
5. Teitelbaum SL, Ross FP (2003)
Genetic regulation of osteoclast development and function. Nat Rev Genet 4:
638-649.
6. Roodman GD (1996) Advances in bone
biology: The osteoclast. Endocr Rev 17: 308-332.
7. Youssef M, Ashkar S, Hamade E,
Gutknecht N, Lampert F, et al. (2007) The effect of low-level laser therapy
during orthodontic movement: A preliminary study. Lasers Med Sci 23: 27-33.
8. Altan BA, Sokucu O, Ozkut MM, Inan
S (2012) Metrical and histological investigation of the effects of low-level
laser therapy on orthodontic tooth movement. Lasers Med Sci 27: 131-140.
9. Fujita S, Yamaguchi M, Utsunomiya
T, Yamamoto H, Kasai K (2008) Low-energy laser stimulates tooth movement
velocity via expression of RANK and RANKL. Orthod Craniofac Res 11: 143-155.
10. Suzuki SS, Garcez AS, Suzuki H,
Ervolino E, Moon W, et al. (2016) Low-level laser therapy stimulates bone
metabolism and inhibits root resorption during tooth movement in a rodent
model. J Biophotonics 9: 1222-1235.
11. Nishijima Y, Yamaguchi M, Kojima
T, Aihara N, Nakajima R, et al. (2006) Levels of RANKL and OPG in gingival
crevicular fluid during orthodontic tooth movement and effect of compression
force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res 9: 63-70.
12. Alikhani M, Raptis M, Zoldan B,
Sangsuwon C, Lee Y, et al. (2013) Effect of micro-osteoperforations on the rate
of tooth movement. Am J Orthod Dentofacial Orthop 144: 639-648.
13. Barbieri G, Solano P, Alarcón JA,
Vernal R, Rios-Lugo J, et al. (2013) Biochemical markers of bone metabolism in
gingival crevicular fluid during early orthodontic tooth movement. Angle Orthod
J 83: 63-69.
14. Grant M, Wilson J, Rock P, Chapple
I (2013) Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic
tooth movement. Eur J Orthod 35: 644-651.
15. Sousa MV, Scanavini MA, Sannomiya
EK, Velasco LG, Angelieri F (2011) Influence of low-level laser on the speed of
orthodontic movement. Photomed Laser Surg 29: 191-196.
16. Doshi-Mehta G, Bhad-Patil WA
(2012) Efficacy of low-intensity laser therapy in reducing treatment time and
orthodontic pain: A clinical investigation. Am J Orthod Dentofacial Orthop 141:
289-297.
17. Seifi M, Shafeei HA, Daneshdoost
S, Mir M (2007) Effects of two types of low-level laser wave lengths (850 and
630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci 22:
261-264.
18. Gama SK, Habib FA, Monteiro JS,
Paraguassú GM, Araújo TM, et al. (2010) Tooth movement after infrared laser phototherapy:
Clinical study in rodents. Photomed Laser Surg 28: S79-83.
19. Marquezan M, Bolognese AM, Araújo
MT (2010) Effects of two low-intensity laser therapy protocols on experimental
tooth movement. Photomed Laser Surg 28: 757-762.
20. Yoshida T, Yamaguchi M, Utsunomiya
T, Kato M, Arai Y, et al. (2009) Low-energy laser irradiation accelerates the
velocity of tooth movement via stimulation of the alveolar bone remodeling.
Orthod Craniofac Res 12: 289-298.
21. Yamaguchi M, Hayashi M, Fujita S,
Yoshida T, Utsunomiya T, et al. (2010) Low-energy laser irradiation facilitates
the velocity of tooth movement and the expressions of matrix
metalloproteinase-9, cathepsin K and alpha (v) beta (3) integrin in rats. Eur J
Orthod 32: 131-139.
22. Cossetin E, Janson G, de Carvalho
MG, de Carvalho RA, Henriques JF, et al. (2013) Influence of low-level laser on
bone remodeling during induced tooth movement in rats. Angle Orthod 83:
1015-1021.
23. Genc G, Kocadereli I, Tasar F,
Kilinc K, El S, et al. (2013) Effect of low-level laser therapy (LLLT) on
orthodontic tooth movement. Lasers Med Sci 28: 41-47.
24. Caccianiga G, Paiusco A, Perillo
L, Nucera R, Pinsino A, et al. (2017) Does low-level laser therapy enhance the
efficiency of orthodontic dental alignment? Results from a randomized pilot study.
Photomed Laser Surg 35: 421-426.
25. Qamruddin I, Alam MK, Mahroof V,
Fida M, Khamis MF, et al. (2017) Effects
of low-level laser irradiation on the rate of orthodontic tooth movement and
associated pain with self-ligating brackets. Am J Orthod Dentofacial Orthop
152: 622-630.
26. Arumughan S, Somaiah S, Muddaiah
S, Shetty B, Reddy G, et al. (2018) A comparison of the rate of retraction with
low-level laser therapy and conventional retraction technique. Contemp Clin
Dent 9: 260-266.
27. Baxter GD, Diamantopoulos C (1995)
Therapeutic lasers: Theory and practice. 1st Edn. New York: Elsevier
Health Sciences, pp: 1-8.
28. Mester E, Mester AF, Mester A
(1985) The biomedical effects of laser application. Lasers Surg Med 5: 31-39.
29. Rody WJ Jr, Wijegunasinghe M,
Wiltshire WA, Dufault B (2014) Differences in the gingival crevicular fluid
composition between adults and adolescents undergoing orthodontic treatment.
Angle Orthod 84: 120-126.
30. Milligan M, Arudchelvan Y, Gong SG
(2017) Effects of two wattages of low-level laser therapy on orthodontic tooth
movement. Arch Oral Biol 80: 62-68.
31. Aihara N, Yamaguchi M, Kasai K
(2006) Low-energy irradiation stimulates formation of osteoclast-like cells via
RANK expression in vitro. Lasers Surg
Med 21: 24-33.
32. Kim SJ, Moon SU, Kang SG, Park YG
(2009) Effects of low-level laser therapy after corticision on tooth movement
and paradental remodeling. Lasers Surg Med 41: 524-533.
33. Domínguez A, Gómez C, Palma JC
(2015) Effects of low-level laser therapy on orthodontics: Rate of tooth
movement, pain and release of RANKL and OPG in GCF. Lasers Med Sci 30: 915-923.
34. Limpanichkul W, Godfrey K, Srisuk
N, Rattanayatikul C (2006) Effects of low-level laser therapy on the rate of
orthodontic tooth movement. Orthod Craniofac Res 9: 38-43.
35. Kansal A, Kittur N, Kumbhojkar V,
Keluskar KM, Dahiya P (2014) Effects of low-intensity laser therapy on the rate
of orthodontic tooth movement: A clinical trial. Dent Res J (Isfahan) 11:
481-488.
36. Yassaei S, Fekrazad R, Shahraki N
(2013) Effect of low level laser therapy on orthodontic tooth movement: A
review article. J Dent Tehran Univ 10.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Diabetes (ISSN: 2644-3031)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- BioMed Research Journal (ISSN:2578-8892)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)