756
Views & Citations10
Likes & Shares
Aim: The aim of this study was to analyze craniofacial morphology by evaluating skeletal cephalometric profiles of HIV-positive adolescents receiving antiretroviral therapy.
Methods: 25 HIV positive adolescent patients aged between 10 and 18 years (study group) were selected and then compared with 25 normoreactive adolescent patients (control group), paired by sex and age. The patients were also catagorised into 3 age ranges (10-12, 13-15 and 16-18 years). Cephalometric tracings of eighteen (linear and angular) measurements on teleradiographs were done by using 2 methodologies. The mean values of each measurement were compared between the two groups by age range.
Results: The majority of measurements checked in HIV-positive adolescents for the 13-15 year age range were diminished. The statistically significant differences (P>0.05) were found only in inclination of the palatal plane (12-14 years) and position of the maxilla in anteroposterior direction (16-18 years).
Conclusion: These results led to conclude that some of the cephalometric measurements of HIV-positive adolescents may be similar to those of the normoreactive subjects
Keywords: Cephalometric, Antiretroviral therapy, Human immunodeficiency virus, Craniofacial
INTRODUCTION
The highly active antiretroviral therapy (HAART) in 1990s, patients with human immunodeficiency virus (HIV1) have undergone an increase in their quality and assumption of life. The adverse effects of combination of these antiretroviral drugs started to be identified through compromised physiologic functions in various systems and organs [1-4]. The changes identified in the pediatric group include mitochondrial toxicity, liver dysfunction, renal toxicity, insulin resistance, hypertension, cardiac dysfunction, increased risk for cardiovascular diseases and decreased bone mineral density [5-15].
Dental treatment of patients with HIV/AIDS was concentrated on diagnosis, epidemiology, and treatment of oral presentations due to immunodepression [16-20]. Hence, it is possible that changes may be verified not only in teeth, but also in craniofacial growth pattern of HIV1 children and adolescents who are undergoing HAART.
Several studies and various authors have illustrated the capacity of medications and chronic systemic diseases to lead to changes in craniofacial growth and development [21-24]. However, no studies of HIV/AIDS patients were found; hence, there is no way to estimate whether disease or its treatment can have impact on craniofacial growth. Recently, studies have pointed out that there are fewer studies about children and adolescents with HIV and most importantly about the adverse effects of HAART on this population [24-26]. In agreement with most recent concerns raised in this particular area of research, the aims and objectives of this study were to analyze craniofacial morphology by evaluating skeletal cephalometric profile of HIV1 adolescent patients, all infected by vertical transmission and hence submitted to antiretroviral therapy and thus to compare them with normoreactive patients. We explained use of a pilot study to substantiate need for longitudinal research.
MATERIAL AND METHODS
A case-control study was conducted with satisfactory sample of consecutive adolescent patients seropositive for HIV and the normoreactive patients who attended a dental hospital for orthodontic treatment during 12 months. The protocol of the study was approved by university research ethical committee. 25 HIV positive adolescent patients aged between 10 and 18 years (study group) were selected and then compared with 25 normoreactive adolescent patients (control group), paired by sex and age. The patients were vertically HIV infected, with positive serology confirmed in 2 different tests and had been given antiretroviral therapy since they were born. The patients were also catagorised into 3 age ranges (10-12, 13-15 and 16-18 years).
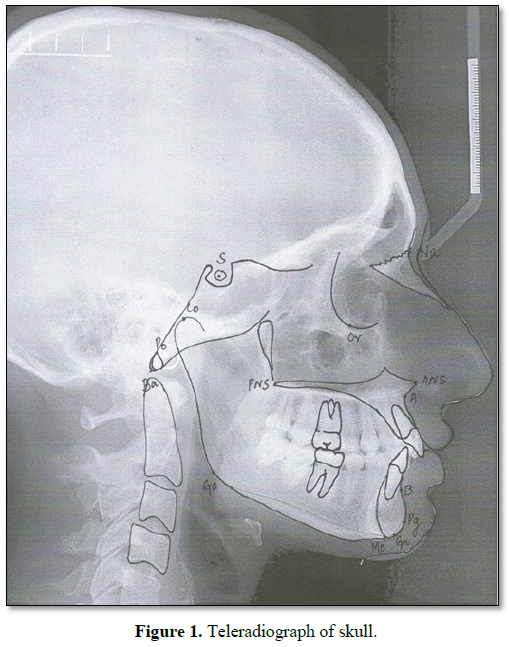
The patients excluded from this study were those undergoing any long-term systemic therapy for severe chronic diseases (except AIDS in study group) who had received earlier radiotherapy, chemotherapy or any previous orthodontic as well as orthopedic treatment. The diagnostic aids for orthodontic documentation were facial photographs, panoramic radiograph, lateral teleradiograph and study models. For this study, we used only teleradiographs, on which cephalometric points of hard profile were identified and used for measuring craniofacial morphology. Cephalometric tracings of eighteen (linear and angular) measurements on teleradiographs were done by using 2 methodologies. The mean values of each measurement were compared between the two groups by age range. Overall, 14 points and 18 (linear and angular) measurements were used, all based on the previous studies by various authors [27-34] (Figure 1 and Table 1).
To decrease measurement errors, cephalometric points and measurements were traced with 2 different methodologies. In the initial (semi-automated) methodology for tracing on cephalograms, a dental radiologist used a Compaq Presario microcomputer (specifications: 1.7 Ghz, 768 Mb RAM, HD 30 GB, Windows XP SP3; Pentium 4; Hewlett Packard, Palo Alto, Ca) and Radiocef Studio software (RadioMemory, Belo Horizonte). The cephalometric landmarks were manually marked and software traced lines and angles, including relevant measurements. In the second (manual) methodology for each radiograph, orthodontist used a transparent acetate sheet (specifications: Ultraphan; 3M Unitek, Monrovia, Calif) measuring 8 3 10 in and 0.003 in thick and a propelling pencil with 0.5 mm thick graphite to mark points, with measurements done manually.
All measurements were listed into a spreadsheet (specifications: Microsoft Office Excel 2007; Microsoft, Redmond, Wash) to get mean values of each angle and linear measurement calculated by semi-automated and manual methodologies. The data obtained for study and control groups were compared. We considered that pubertal growth spurt in girls occurred from 10 years of age [35], reaching its maximum at 12 years [36] whereas in boys it started from 9 years [35] and reached its peak at 14 years [36]. Hence, the time interval between 9 and 12 years was chosen as main growth spurt period for both sexes. Therefore, 2 other age ranges were defined: before growth spurt (6-8 years old) and after growth spurt (13-17 years old).
The data were analyzed by using the Epiinfo software [7] and Bartlett test was performed to verify the homogeneity of variances (P>0.05). For variables without a normal distribution, Wilcoxon test was applied. The significance level was put down at 0.05 or 5%. To determine reliability of agreement between 2 measurement methodologies, intraclass correlation coefficient was used for the statistical analysis.
RESULTS
Twenty five subjects were evaluated in the study group, of which 10 were girls and 15 were boys, all aged between 10 and 18 years (mean age, 14 years). All had been diagnosed with HIV since birth and treated with anti-retroviral therapy from the first year of life. During the time of clinical evaluation, mean count of CD41 T lymphocytes in study group was 752 cells per cubic millimeter (minimum, 180 cells/mm3 and maximum, 1727 cells/mm3). 2 patients had no undetected viral load (5415 and 41,400 copies). These patients were also the ones to have CD41 T lymphocyte counts out of normal range (273 and 180 cells/mm3). Four used front-line drugs composed of 2 nucleoside reverse transcriptase inhibitors and 1 non-nucleoside reverse transcriptase inhibitor. 12 patients used second-line combinations (2 nucleoside reverse transcriptase inhibitors and 1 protease inhibitor with ritonavir booster). 2 patients used 2 nucleoside reverse transcriptase inhibitors plus 1 protease inhibitor.
3 patients used following combinations: 2 nucleoside reverse transcriptase inhibitors and 1 protease inhibitor along with ritonavir booster plus 1 protease inhibitor; 2 nucleoside reverse transcriptase inhibitors with 1 protease inhibitor and 1 integrase inhibitor; 3 nucleoside reverse transcriptase inhibitors and 1 protease inhibitor with ritonavir booster. With reference to methodologies used for calculating cephalometric measurements, we noticed that methods were in agreement to each other for almost all variables and increased intraclass correlation coefficient values (Table 2).
The exceptions were variables Co-A, SN.ANSPNS and SNB, which had good agreement of 60%. Nevertheless, we declared that agreement was positive for SN.ANSPNS and SNB, since P values were statistically significant (P<0.05). As for Co-A, P value was out of significance range (Table 3).
The cephalometric measurements of study and control groups were compared according to various age groups, as mentioned in Table 4.
In 10-12 year age group, when considering mean values, positions of maxilla and mandible in study group were retruded in relation to base of the skull when compared with control group. Growth pattern in study group was more horizontal and effective size of bone bases was increased than in control group. However, the differences were not statistically significant, except for palatal plane inclination (Table 3).
Table 4 shows comparison of cephalometric means with regard to mean values in the 13-15 year age group, the maxilla was retruded slightly, and mandible was protruded in the study group in relation to the base of the skull compared with control group. Also, the former had a decreased effective maxillary size and an increased effective mandibular size than the latter. In this age group, growth patterns were almost similar in both groups. None of the values related to maxillary and mandibular positions in antero-posterior direction, growth pattern and effective linear measurements in this particular age group showed statistically significant differences between study and control groups (Table 4).
With regards to the mean values in 16-18 year age group, the position of maxilla in the study group was slightly decreased, and the mandible was protruded in relation to base of the skull when compared with the control group. Although the former had an effective maxillary size smaller than that of latter, effective mandibular size was similar between 2 groups. The growth pattern was more horizontal in the study group than in the control group. The only statistically significant difference was position of the maxilla in anteroposterior direction (SNA) (Table 5).
DISCUSSION
Adolescents seropositive for HIV now attend the dental hospitals and demand full treatment for their oral health conditions. Studies evaluating growth and development of face have identified that, up to 5 years of age well-developed craniometric dimensions and especially significant increase in the height and width of the jaw are observed. But the greatest gain in growth occurs only after 6 years of age, with continuous increase in jaw length and facial height, width, and depth, until craniofacial dimensions reach maturity during adolescence, between 13 and 15 years of age [37]. Because of the drawbacks inherent in cross-sectional study, differences that could be found in different age groups in our study which suggest continuous craniofacial growth, with whole face growing vertically and horizontally in both groups.
In these results, we found that 2 measurements had statistically significant differences: the angle between palatal plane and base of skull (SN.ANSPNS) and angle demonstrating position of the maxilla in antero-posterior direction (in relation to the base of skull), represented by SNA. In 10-12 year age range, the SN.ANSPNS values showed the rotation of maxilla (palatal plane) was increased in the HIV1 patients, but these values diminished in subsequent age groups, along with the values of normoreactive patients. No significant changes in completion of growth were observed; this occurred in patients in our study group.
The angle SNA was shown to be significantly decreased in the 13-15 and 16-18 year age group. The reduction in measurement was interpreted as retrusion of maxillary bone [35]. Although the study was not longitudinal, there were 3 interpretations for this craniofacial change. The first was this change could be a consequence of the respiratory pattern of HIV1 adolescents, which is compromised by s airway infections during growth period. SNA may be decreased in those with compromised respiratory function of upper airway, as in mouth-breathing patients [31,32].
The HIV infection associated or not with states of immune activation and inflammatory processes, can change osteoclastogenesis by increasing rate of apoptosis of primary osteoblasts, decreasing calcium deposition and alkaline phosphatase activity, diminishing specific bone proteins and compromising differentiation of mesenchymal cells into osteoblasts [33]. The long-term use of HAART may be definitely responsible for systemic alterations that affect growth of these adolescent children [5,7,12,13].
HAART emerged as a solution to deleterious effects caused by the virus by lowering the circulating viral load. The immunologic reconstitution induced by use of antiretroviral agents, expressed by increase in CD41 T lymphocytes, has allowed these patients to be clinically stable, with reductions in opportunist infections that could bring about malnutrition. Although gains in weight and height among HIV1 patients undergoing HAART are vital, these gains are less than weight and height gains of normoreactive adolescents [36,37]. In this study, other linear and angular values of HIV1children had no statistically significant differences when compared with the control group, However, it was possible to identify a trend toward decrease in the linear measurements of maxilla, mandible and base of the skull in the study patients between 13-15 and 16-18 years old. In this age group 14 angular and linear measurements in our study patients were lower than those of the controls. This difference was seen only in 5 measurements in the 10-12 year age group and in 7 measurements in the 13-15 year age group. We believe that the findings could be a basis for longitudinal study with these group patients. Nevertheless, this study was a cross-sectional and did not establish cause and effect relationship; this could be a drawback of our study.
Another limitation and confounding factor of study was that only teleradiographs of adolescents who were referred for the orthodontic treatment were included and consequently they had some amount of deviation from normality (in both groups). An ideal study design would include all adolescents, irrespective of their need for orthodontic treatment. But, ethical questions are raised as a result of submitting adolescents to ionizing radiation not only during growth period, but also at various times throughout life, only to see possible changes in their craniofacial growth. It was not possible to mention that either HIV or HAART alone is responsible for possible developmental delay, or to evaluate HIV1 patients with and without use of HAART separately, since in India all HIV1children who are under medical treatment are also receiving antiretroviral treatment. Although 18 comparisons per group were done, only 2 measurements had statistically significant differences with marginally significant P value. Since no overall differences were found between 2 groups in our study, we hypothesized that beneficial effects of antiretroviral therapy overcame adverse effects.
CONCLUSION
The majority of measurements in HIV1 children and adolescents were not different from the control group to generate statistically significant difference in craniofacial growth.
1. Abrescia N, D'Abbraccio M, Figoni M, Busto A, Maddaloni A, et al. (2005) Hepatotoxicity of antiretroviral drugs. Curr Pharm Des 11: 3697-710.
2. Gortmaker S, Hughes M, Cervia J (2001) Effects of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med 34521: 1522-1528.
3. Jain RG, Furfine ES, Pedneault L, White AJ, Lenhard JM (2001) Metabolic complications associated with antiretroviral therapy. Antiviral Res 51: 151-77.
4. De martino M, Tovo PA, Balducci M (2000) Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA 248: 190-197.
5. Liu K, Sun Y, Liu D, Yin J, Qiao L, et al. (2013) Mitochondrial toxicity studied with the PBMC of children from the Chinese national pediatric highly active antiretroviral therapy cohort. PLoS One.
6. Miro O, Villarroya J, Garrabou G, Lopez S, Rodrıguez de la Concepcion M, et al. (2005) In vivo effects of highly active antiretroviral therapies containing the protease inhibitor nelfinavir on mitochondrially driven apoptosis. Antivir Ther 10: 945-951.
7. Bwakura-Dangarembizi M, Musiime V, Szubert AJ, Prendergast AJ, Gomo ZA, et al. (2015) Prevalence of lipodystrophy and metabolic abnormalities in HIV-infected African children after 3 years on first-line antiretroviral therapy. Pediatr Infect Dis J.
8. Chiappini E, Galli L, Tovo PA (2007) Cancer rates after year 2000 significantly decreases in children with perinatal HIV infection: A study by the Italian Register for HIV Infection in Children. J Clin Oncol 25: 97-101.
9. Innes S, Abdullah KL, Haubrich R, Cotton MF, Browne SH (2016) High prevalence of dyslipidemia and insulin resistance in HIV-infected pre-pubertal African children on antiretroviral therapy. Pediatr Infect Dis J 35: e1-7.
10. Aurpibul L, Bunupuradah T, Sophan S, Boettiger D, Wati DK, et al. (2015) Prevalence and incidence of liver dysfunction and assessment of biomarkers of liver disease in HIV-infected Asian children. Pediatr Infect Dis J 34: e153-158.
11. Chatterton-Kirchmeier S, Camacho-Gonzalez AF, McCracken CE, Chakraborty R, Batisky DL (2015) Increased prevalence of elevated blood pressures in HIV-infected children, adolescents and young adults. Pediatr Infect Dis J 34: 610-614.
12. Noguera A, Fortuny C, Munoz-Almagro C (2004) Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics 114: e598-603.
13. Namuyonga J, Lubega S, Musiime V, Lwabi P, Lubega I, et al (2016) Cardiac dysfunction among Ugandan HIV-infected children on antiretroviral therapy. Pediatr Infect Dis J 35: e85-88.
14. Sainz T, Alvarez-Fuente M, Navarro ML, Dıaz L, Rojo P, et al. (2014) Madrid cohort of HIV-infected children and adolescents integrated in the pediatric branch of the Spanish National AIDS Network (CoRISPE). Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: the CaroVIH Study. J Acquir Immune Defic Syndr 65: 42-49.
15. Cruz ML, Cardoso CA (2015) Perinatally infected adolescents living with human immunodeficiency virus (perinatally human immunodeficiency virus). World J Virol 4: 277-284.
16. Stagi S, Galli L, Cecchi C, Chiappini E, Losi S, et al. (2010) Final height in patients perinatally infected with human immunodeficiency virus. Horm Res Paediatr 74: 165-171.
17. Ortega KL, Rezende NP, Lotufo MA, Magalhaes MH (2008) Mandibular lesion in HIV-positive patient. J Oral Maxillofac Surg 66: 2140-2144.
18. Ortega KL, Rezende NP, Magalhaes MH (2009) Diagnosing secondary syphilis in a patient with HIV. Br J Oral Maxillofac Surg 47: 169-170.
19. Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM (2013) Metabolic complications and treatment of perinatally HIV infected adolescents. J Int AIDS Soc 16: 18600.
20. Ortega KL, Vale DA, Magalhaes MH (2009) Impact of PI and NNRTI HAART-based therapy on oral lesions of Brazilian HIV-infected patients. J Oral Pathol Med 38: 489-494.
21. Francischini E, Martins FM, Braz-Silva PH, Magalhaes MH, Ortega KL.(2010) HIV-associated oral plasmablastic lymphoma and role of adherence to highly active antiretroviral therapy. Int J STD AIDS 21: 68-70.
22. Oliveira MA, Gallottini M, Pallos D, Maluf PS, Jablonka F, et al. (2011) The success of endosseous implants in human immunodeficiency virus-positive patients receiving antiretroviral therapy: A pilot study. J Am Dent Assoc 142: 1010-1016.
23. Guerreiro da Silva Junior N, Pedreira EN, Tuji FM, Warmling LV, Ortega KL (2014) Prevalence of calcified carotid artery atheromas in panoramic radiographs of HIV-positive patients undergoing antiretroviral treatment: A retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol 117: 67-74.
24. Hsieh YJ, Darvann TA, Hermann NV, Larsen P, Liao YF, et al. (2016) Facial morphology in children and adolescents with juvenile idiopathic arthritis and moderate to severe temporomandibular joint involvement. Am J Orthod Dentofacial Orthop 149: 182-191.
25. Trigueiro M, Tedeschi-Oliveira SV, Melani RF, Ortega KL (2010) An assessment of adverse effects of antiretroviral therapy on development of HIV positive children by observation of dental mineralization chronology. J Oral Pathol Med 39: 35-40.
26. El-Bialy T, Aboul-Azm SF, El-Sakhawy M (2000) Study of craniofacial morphology and skeletal maturation in juvenile diabetics (type I). Am J Orthod Dentofacial Orthop 118: 189-195.
27. Sadeghianrizi A, Forsberg CM, Marcus C, Dahll€of G (2005) Craniofacial development in obese adolescents. Eur J Orthod 27: 550-555.
28. Orup HI Jr, Holmes LB, Keith DA, Coull BA (2003) Craniofacial skeletal deviations following in utero exposure to the anticonvulsant phenytoin: Monotherapy and polytherapy. Orthod Craniofac Res 6: 2-19.
29. Amini F, Jafari A, Eslamian L, Sharifzadeh S (2007) A cephalometric study on craniofacial morphology of Iranian children with beta thalassemia major. Orthod Craniofac Res 10: 36-44.
30. Gjorup H, Kjaer I, Sonnesen L, Haubek D, Beck-Nielsen SS, et al. (2011) Craniofacial morphology in patients with hypophosphatemic rickets: Cephalometric study focusing on differences between bone of cartilaginous and intramembranous origin. Am J Med Genet A 155A: 2654-2660.
31. Fjeld MG, Arvidsson LZ, Stabrun AE, Birkeland K, Larheim TA, et al. (2009) Average craniofacial development from 6 to 35 years of age in mixed group of patients with juvenile idiopathic arthritis. Acta Odontol Scand 67: 153-160.
32. Al-Thomali Y, El-Bialy TH (2012) Cephalometric craniofacial features of growing patients with chronic renal failure. Arch Oral Biol 57: 257-263.
33. Niles DG, Rynearson RD, Baum M, Neufeld RD, Caruso JM (2000) A study of craniofacial growth in infant heart transplant recipients receiving cyclosporine. J Heart Lung Transplant 19: 231-239.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- International Journal of Diabetes (ISSN: 2644-3031)