768
Views & Citations10
Likes & Shares
INTRODUCTION
Bones, heart, liver, lungs and brain disorders are the major concern of
human overall health across the globe. The World Health Organization (WHO)
estimates, in 2016, ~17.5 million people die due to cardiovascular (heart)
disorders, ~3.5 million people die due to lungs disorders, ~1.3 million people
die due to liver disorders around the globe each year [1]. Moreover, ~1.2
million people most frequently diagnosed adult-onset brain disorders in each
year in the USA [2]. Three main criteria to keep a healthy heart include the
opening blood vessels, strengthening the heart muscle and controlling free
radical damage by antioxidants [3]. The release of liver mitochondrial enzymes
is considered strong evidence for hepatic (liver) necrosis, which is associated
with an increased production of reactive oxygen species (ROS) that leads to
hepatic lipid peroxidation [4-6]. Oxidative stress in the respiratory system
increases the production of mediators of pulmonary inflammation and initiate or
promote mechanisms of carcinogenesis [7]. The lung is one of the major organs,
which is highly exposed by various oxidants, i.e., endogenous and exogenous
oxidants (cigarette smoke, mineral dust, ozone and radiation). These oxidants
produce free radicals, while reactive oxygen species (ROS) and reactive
nitrogen species (RNS) are produced by phagocytes as well as by alveolar,
polymorph nuclear, bronchial and different endothelial cells [8]. However, the
role of oxidative stress in the pathogenesis of lung diseases has been widely
reported such as asthma, chronic obstructive pulmonary disease (COPD), lung
malignancies and parenchymal lung diseases like idiopathic pulmonary fibrosis
and lung granulomatous diseases [9]. Serotonin (5-hydroxytryptamine, 5-HT) is
among the brain’s neuromodulators responsible for behavior and understanding
[10]. Apart from medicines, non-pharmacologic methods that can increase
serotonin by increasing recognition and happiness and well-being. These factors
can protect against mental and physical disorders [11]. There is currently no
universally accepted test formulation, which improve the organ health
biomarkers. With this respect, the novel test formulation was designed on the
basis of best scientific literature, which is the combination of herbal
products viz. Panax ginseng extract and beta carotene, minerals viz.
calcium chloride, magnesium gluconate, zinc chloride, sodium selenate, ferrous
sulfate and vitamins viz. vitamin B12, vitamin D3, ascorbic acid and
vitamin B6. This formulation is designed for overall functioning of the organs
that can results in improved overall health conditions against many
pathological conditions such as lung disorder, liver disorder, breast cancer,
liver cancer, aging, muscle damage and overall health. Minerals and vitamins
present in the test formulation provide significant functional support to all
the vital organs [12-14]. In addition, Panax ginseng is one of the best
reported medicinal plants that improve mental, physical abilities, cognitive
health and is potent immune modulator [15,16].
Various study data suggested the effect of Energy Therapy in cancer
patients through therapeutic touch [17]; massage therapy [18], etc.
Complementary and Alternative Medicine (CAM) therapies are preferred model of
treatment, among which Biofield Therapy (or Healing Modalities) is one approach
to enhance emotional, mental, physical and human wellness. The National Center
of Complementary and Integrative Health (NCCIH) has recognized and allowed
Biofield Energy Healing as a CAM approach in addition to other therapies and
medicines such as natural products, chiropractic/osteopathic manipulation, Qi
Gong, deep breathing, Tai Chi, yoga, meditation, massage, special diets,
healing touch, relaxation techniques, traditional Chinese herbs and medicines,
naturopathy, movement therapy, homeopathy, progressive relaxation, guided
imagery, pilates, acupuncture, acupressure, Reiki, rolfing structural
integration, hypnotherapy, Ayurvedic medicine, mindfulness, essential oils,
aromatherapy and cranial sacral therapy. The Human Biofield Energy has subtle
energy that has the capacity to work in an effective manner [19]. CAM therapies
have been practiced worldwide with reported clinical benefits in different
health disease profiles [20]. This energy can be harnessed and transmitted by
the practitioners into living and non-living things via the process of Biofield
Energy Healing. The Biofield Energy Treatment, the Trivedi Effect®,
has been reported to have a significant impact in the field of cancer research
[21,22], materials science [23,24], microbiology [25,26], agriculture [27,28],
nutraceuticals [29,30] and biotechnology [31,32]. Further, the Trivedi Effect®
also significantly improved bioavailability of various low bioavailable
compounds [33-35], an improved overall skin health [36,37], bone health
[38-40], human health and wellness. Based on the excellent outcomes of the
Biofield Energy Therapy in wide spectrum of areas, the authors intend to see
the impact of the Biofield Energy Healing Treated test formulation on the
function of vital organs such as bones, heart, liver, lungs and brain specific
biomarkers in different cell-lines.
MATERIALS AND
METHODS
Chemicals and
reagents
Zinc chloride, magnesium gluconate, β-carotene and calcitriol were
purchased from TCI chemicals, Japan. Ferrous sulfate, vitamin B6, vitamin D3,
vitamin B12, calcium chloride, naringenin, trimetazidine (TMZ),
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) and
ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical Co.
(St. Louis, MO). Silymarin and curcumin were obtained from Sanat Chemicals,
India and quercetin obtained from Clearsynth, India. Panax ginseng
extract obtained from panacea Phytoextracts, India. Sodium selenate and
ascorbic acid were obtained from Alfa Aesar, India. Reverse Transcription Kit,
RNeasy Mini Kit and Syber Green PCR kits were procured from Quagen, India. All
the other chemicals used in this experiment were analytical grade procured from
India.
Biofield energy
healing strategy
The test formulation was the combination of eleven ingredients viz.
calcium chloride, Panax ginseng extract, vitamin B12, β-carotene,
vitamin D3, zinc chloride, magnesium gluconate, sodium selenate, ferrous
sulfate, ascorbic acid and vitamin B6. The test formulation and the cell media
was divided into two parts; one untreated (UT) and other part received the
Biofield Energy Treatment remotely by a renowned Biofield Energy Healer, Thomas
Charles Slade, USA, under laboratory conditions for ~3 min through healer’s
unique Biofield Energy Transmission process and was labeled as the Biofield
Energy Treated (BT) test formulation/media. Further, the untreated group was
treated with “sham” healer for comparison purpose. The “sham” healer did not
have any knowledge about the Biofield Energy Healing Treatment. Biofield Energy
Healer was located in the USA; however the test items were located in the
research laboratory of Dabur Research Foundation, New Delhi, India. Biofield
Energy Healer in this experiment did not visit the laboratory, nor had any
contact with the test samples. After that, the Biofield Energy Treated and
untreated test items were kept in similar sealed conditions and used for the
study as per the study plan.
Assessment of cell
viability using MTT assay
Cells were counted using hemocytometer and plated in 96-well plates at
the specific density described in Table 1. The cells were then incubated
overnight under growth conditions and allow to cell recovery and exponential
growth. Following overnight incubation, cells were treated with different
concentrations of test formulations (BT/UT). Following respective treatments,
cells were incubated in a CO2 incubator at 37°C, 5% CO2
and 95% humidity and incubated for time period mentioned in Table 1.
After incubation, the plates were taken out and 20 µL of 5 mg/mL of MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide solution was added
to all the wells followed by additional incubation for 3 h at 37°C. The
supernatant was aspirated and 150 µL of DMSO was added to each well to dissolve
formazan crystals. The absorbance of each well was read at 540 nm using Synergy
HT microplate reader. The percentage cytotoxicity at each tested concentration
of TI was calculated using Equation 1:
% Cytotoxicity = [(R-X)/R] * 100 (1)
Where, X=Absorbance of treated cells; R=Absorbance of untreated cells
Evaluation of the cytoprotective effect of the
formulation
Cells (human cardiac fibroblasts-HCF; human hepatoma cells-HepG2; and
adenocarcinomic human alveolar basal epithelial cells-A549) were counted and
plated in suitable medium followed by overnight incubation. The cells were then
treated with the test items/positive control at the non-cytotoxic
concentrations for 24 h. After 24 h, oxidative stress was given to the cells
using 10 mM t-BHP for 3.5 h. The untreated cells served as a control
that did not receive any treatment and was maintained in cell growth medium
only. Cells treated with 10 mM of t-BHP alone served as negative
control. After 3.5 h of incubation with t-BHP the above plates were
taken out and cell viability was determined by MTT assay. The percentage
protection corresponding to each treatment was calculated using Equation 2:
% Protection =
[(Absorbancesample-Absorbancet-BHP)]*100/ [Absorbanceuntreated-Absorbancet_BHP] (2)
Assessment of
alkaline phosphatase (ALP) activity
The cells (human bone osteosarcoma cells-MG-63 and human endometrial
adenocarcinoma cells-Ishikawa) were counted using an hemocytometer and plated
in 24-well plates at the density corresponding to 1 × 104 cells/well
in phenol-free DMEM supplemented with 10% CD-FBS. Following the respective
treatments, the cells in the above plate were incubated for 48 h in CO2
incubator at 37°C, 5% CO2 and 95% humidity. After 48 h of
incubation, the plates were taken out and processed for the measurement of ALP
enzyme activity. The cells were washed with 1x PBS and lysed by freeze-thaw
method, i.e., incubation at -80°C for 20 min followed by incubation at 37°C for
10 min. To the lysed cells, 50 µL of substrate solution, i.e., 5 mM of p-nitrophenyl
phosphate (pNPP) in 1 M diethanolamine and 0.24 mM magnesium chloride
(MgCl2) solution (pH 10.4) was added to all the wells followed by
incubation for 1 h at 37°C. The absorbance of the above solution was read at
405 nm using Synergy HT microplate reader (Biotek, USA). The absorbance values
obtained were normalized with substrate blank (pNPP solution alone)
absorbance values. The percentage increase in ALP enzyme activity with respect
to the untreated cells (baseline group) was calculated using Equation 3:
% Increase in ALP =
{(X-R)/R}*100 (3)
Where, X=Absorbance of cells corresponding to positive control and test
groups; R=Absorbance of cells corresponding to baseline group (untreated cells)
Estimation of
lactate dehydrogenase (LDH) in human cardiac fibroblasts (HCF)
The human cardiac fibroblasts (HCF) cells were counted and plated at
the density of 0.25 × 106 cells/ well in 24-well plates in cardiac
fibroblast specific medium followed by overnight incubation. The cells were
then treated with the test formulation/positive control at the non-cytotoxic
concentrations for 24 h. After 24 h, oxidative stress was given to the cells
using 10 mM t-BHP for 3.5 h. The untreated cells were served as control
that did not receive any treatment and were maintained in cell growth medium
only. Cells treated with 10 mM of t-BHP alone served as the negative
control. After 3.5 h of incubation with t-BHP the above plates were
taken out and LDH activity was determined using LDH activity kit as per
manufacturer’s instructions. The percent increase in LDH activity was
calculated using Equation 4.
%
Increase = [(LDH activitysample-LDH activityt-BHP)]*100/
[LDH activityuntreated-LDH activityt_BHP] (4)
Estimation of ALT in liver cells (HepG2)
The human hepatoma cells (HepG2) were counted
and plated
at the density of 5 × 104 cells/well in 48-well plates in DMEM
media followed by overnight incubation. The cells were then treated with the test
formulation/positive control at the non-cytotoxic concentrations for 24 h.
After 24 h, oxidative stress was given to the cells using 400 µM t-BHP for 3.5 h. The untreated cells
served as control that did not receive any treatment and were maintained in
cell growth medium only. Cells treated with 400 µM of t-BHP alone served as negative control. After 3.5 h of incubation
with t-BHP the above plates were
taken out and ALT activity was determined using ALT activity kit as per
manufacturer’s instructions. The percent increase in ALT activity was
calculated using Equation 5.
%
Increase = [(ALT activitysample-ALT activityt-BHP)]*100/
[ALT activityuntreated-ALT activityt_BHP] (5)
Estimation
of superoxide dismutase (SOD) in lung (A549) cells
The adenocarcinomic human alveolar basal
epithelial cells (A549) were counted and plated at the density of 1 ×
104 cells/well in 24-well plates in DMEM followed by
overnight incubation.
The cells were then treated with the test formulation/positive control at the
non-cytotoxic concentrations along with 100 µM t-BHP to induce oxidative stress. The untreated cells served as
control that did not receive any treatment and were maintained in cell growth
medium only. Cells treated with 100 µM of t-BHP
alone served as negative control. After 24 h of incubation with t-BHP the above plates were taken out
and SOD activity was determined using SOD activity kit as per manufacturer’s
instructions. The percent increase in SOD activity was calculated using
Equation 6:
%
Increase in SOD activity
= ((X-R)/R)*100 (6)
Where, X=SOD activity corresponding to test item or positive control; R=SOD
activity corresponding to control group
Estimation
of serotonin in neuronal cells (SH-SY5Y)
The human neuroblastoma (SH-SY5Y) cells were
counted and plated at the density of 10 × 104
cells/well in 96-well plates followed by overnight incubation. The cells were then treated with
the test items/positive control at the non-cytotoxic concentrations. The
untreated cells served as control that did not receive any treatment and were
maintained in cell growth medium only. The treated cells were incubated for 24
h. Serotonin release was determined by ELISA as per manufacturer’s protocol. The
percent increase in serotonin levels was calculated using Equation 7.
[(X-R)/R]*100 (7)
Where, X=Serotonin levels corresponding to
test item or positive control; R=Serotonin levels corresponding to control
group
Effect of test
formulation on vitamin D receptor (VDR) in bone (MG-63) cells
The human bone osteosarcoma (MG-63) cells were counted using
hemocytometer were plated at a density of 2 × 105 cells/well in
6-well plates followed by overnight incubation. The cells were then sera starved
for 24 h and treated with the test formulation/positive control at the
non-cytotoxic concentrations. The untreated cells were served as control that
did not receive any treatment and were maintained in cell growth medium only.
The treated cells were incubated for 24 h and VDR expression was determined by
Q-PCR using VDR specific primers. Cells were harvested by scrapping and washed
with PBS. Cell pellets obtained were analyzed for VDR gene expression using
human VDR specific primers: Forward: 5’-GCTGACCTGGTCAGTTACAGCA-3’, Reverse:
5’-CACGTCACTGACGCGGTACTT-3’. VDR gene expression was normalized using
House-keeping (HK) reference. Relative quantification (RQ) of VDR gene in
Biofield Energy Treated cells was calculated with respect to the untreated
cells using Equation 8:
RQ = 2-N (8)
Where N is the relative Threshold Cycle (CT)
value of treated sample with respect to the untreated sample.
STATISTICAL
ANALYSIS
All the values were represented as Mean ± SD
(standard deviation) of three independent experiments. The statistical analysis
was performed using Sigma Plot statistical software (v11.0). For two groups
comparison Student’s t-test was used.
For multiple group comparison, one-way analysis of variance (ANOVA) was used
followed by post-hoc analysis by Dunnett’s test. Statistically significant
values were set at the level of p ≤
0.05.
RESULTS AND DISCUSSION
Cell
viability using MTT assay
Determination of non-cytotoxic concentration
of the test formulation and positive controls by MTT cell viability assay was
used in terms of percent viable cells in six (6) different cell-lines viz. MG-63, Ishikawa, A549, HepG2, HCF
and SH-SY5Y. Based on the percent cell viability data, it was observed that the
formulation and positive controls were safe and non-toxic at the tested
concentrations in six different cell lines and selected for other parameters
analysis.
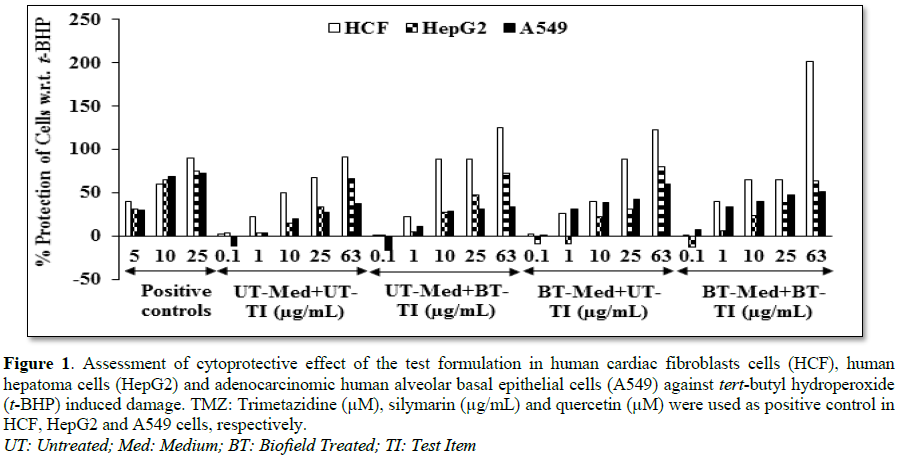
Evaluation of cytoprotective effect
of the test formulation
Estimation of
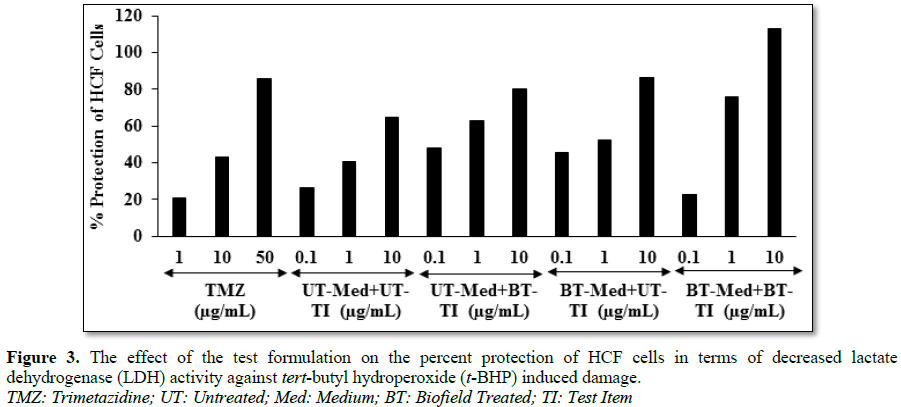
lactate dehydrogenase (LDH) activity in human cardiac fibroblasts (HCF)
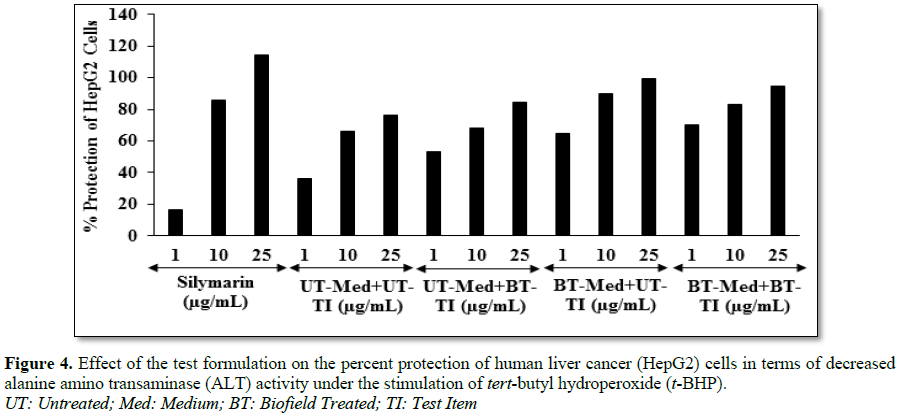
Estimation of alanine amino transferase (ALT) activity in HepG2 cells
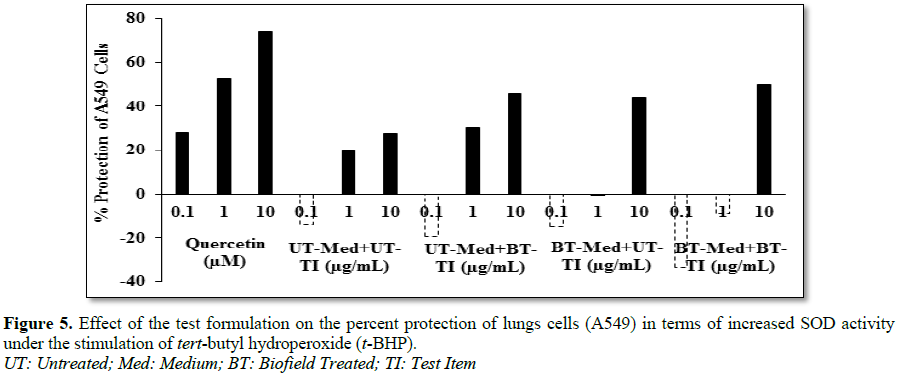
Estimation of
superoxide dismutase (SOD) activity in adenocarcinomic human alveolar basal
epithelial cells (A549)
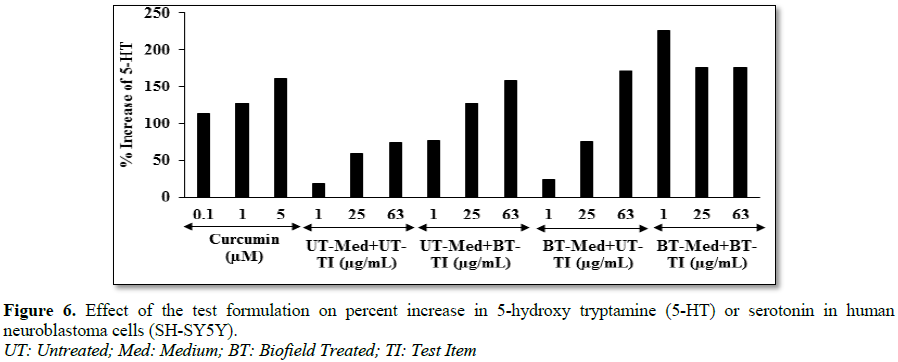
Effect
of test formulation on serotonin in human neuroblastoma (SH-SY5Y) cells
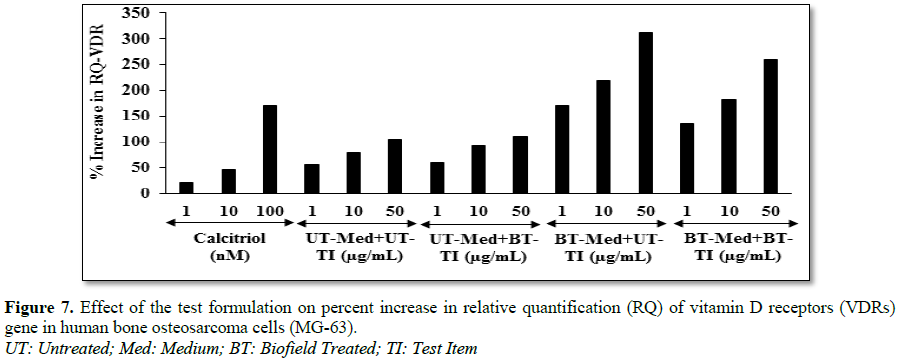
Effect of test
formulation on vitamin D receptors (VDRs)
CONCLUSION
The study outcomes
showed that the tested novel formulation was safe and non-toxic based on cell
viability assay (MTT) in six different tested cells. The UT-Med + BT-TI group
showed 74.4% restoration of cell viability at 10 µg/mL in human cardiac fibroblasts
cells (HCF) compared to the UT-Med + UT-TI group. Moreover, the BT-Med + BT-TI
group showed and 87.5% (at 1 µg/mL) restoration of cell viability in human
hepatoma cells (HepG2) compared to the untreated group. Besides, 209.5%, 757.8%
and 836.2% restoration of cell viability was observed in adenocarcinomic human
alveolar basal epithelial cells (A549) by UT-Med + BT-TI, BT-Med + UT-TI and
BT-Med + BT-TI groups, respectively at 1 µg/mL as compared to the untreated
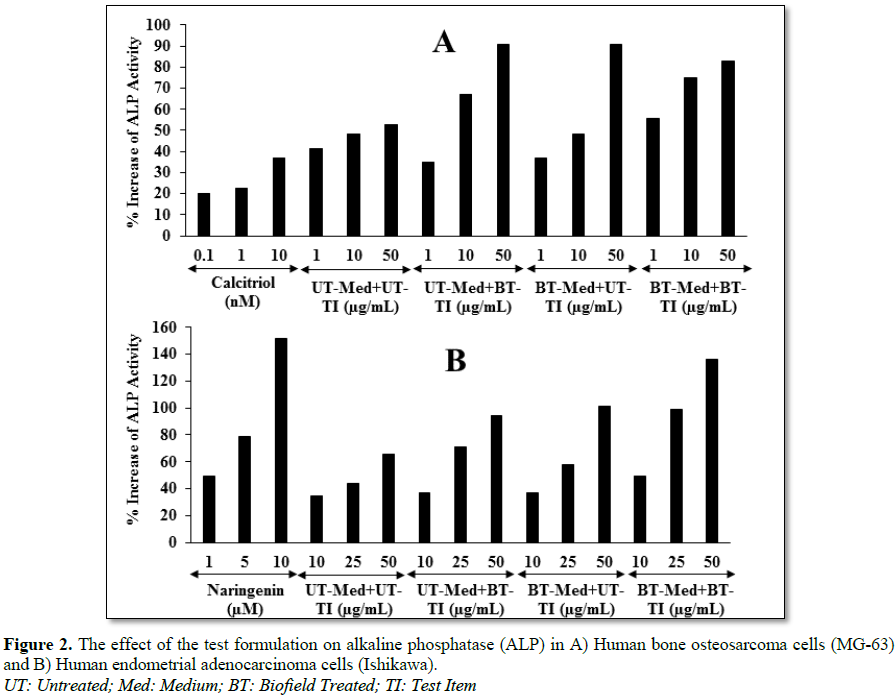
group. Alkaline phosphatase (ALP) activity was significantly increased by 71.7%
and 71.9% in the
UT-Med + BT-TI and BT-Med + UT-TI groups, respectively at 50 µg/mL in human
endometrial adenocarcinoma cells (Ishikawa). The percent protection of HCF
cells (decreased of LDH activity) was significantly increased by 82.8% (at 0.1
µg/mL) and 88.3% (at 1 µg/mL) in the UT-Med + BT-TI and BT-Med + BT-TI groups,
respectively as compared to the untreated group in HCF cells. The percent
protection of HepG2 cells (decreased of ALT activity) was significantly increased
by 79.8% and 94% in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively
at 1 µg/mL compared to the untreated group in HepG2 cells. The percent
protection of A549 (lungs) cells (increased of SOD activity) was significantly
increased by 137% and 80.7% in the BT-Med + BT-TI group at 0.1 and 10 µg/mL,
respectively compared to the untreated group in A549 cells. Serotonin level was
significantly increased by 317.9% and 225.7% in the UT-Med + BT-TI and BT-Med +
BT-TI groups, respectively at 1 µg/mL compared to the untreated group in human
neuroblastoma cells (SH-SY5Y). The relative quantification (RQ) of vitamin D receptors (VDRs) level was
significantly increased by 195.3% (at 1 µg/mL), 176.2% (at 10 µg/mL) and 194.7%
(at 50 µg/mL) in the BT-Med + BT-TI group compared to the untreated
group in MG-63 cells. Taking everything
into account, the Biofield Energy Treatment significantly improved
heart, liver, bones, neuronal and lungs functional enzyme biomarkers and also
protected hepatocyte, cardiomyocyte, pneumocyte, osteocytes and nerve cells
from oxidative damage induced by tert-butyl
hydroperoxide (t-BHP). Thus, it can
be used as a complementary and alternative treatment for the prevention of various types of cardiac disorders (high
blood pressure, congestive heart failure, stroke, peripheral artery disease,
rheumatic heart disease, valvular heart disease, carditis, congenital heart
disease and venous thrombosis, thromboembolic disease, etc.), hepatic disorders
(cirrhosis, liver cancer, hemochromatosis, Wilson disease) and lungs disorders (Asthma, Chronic
bronchitis, Emphysema, Cystic fibrosis, Pneumonia). Further, it could be
useful to improve cell-to-cell messaging, normal cell growth and
differentiation, cell cycling and proliferation, neurotransmission, skin
health, hormonal balance, immune and cardiovascular functions. Moreover, it can
also be utilized in organ transplants (i.e., kidney, liver and heart
transplants), hormonal imbalance, aging and various inflammatory and
immune-related disease conditions like Alzheimer’s Disease (AD), Ulcerative
Colitis (UC), Dermatitis, Asthma, Irritable Bowel Syndrome (IBS), Pernicious Anemia, Multiple Sclerosis, Aplastic
Anemia, Hepatitis, Graves’ Disease,
Diabetes, Parkinson’s Disease, Myasthenia
Gravis, Atherosclerosis, Systemic Lupus
Erythematosus (SLE), stress, etc., to improve overall health and Quality
of Life.
ACKNOWLEDGEMENT
1.
Global Burden of Disease Collaborative Network
(2017) Global Burden of Disease Study 2016 (GBD 2016) Results. Seattle, United States:
Institute for Health Metrics and Evaluation (IHME).
2.
Pal S (2018) Incidence and prevalence of major
neurologic disorders. US Pharm 43: 24.
3.
Rakesh S, Arunporn I (2017) Herbal supplements or
herbs in heart disease: Herbiceutical formulation, clinical trials, futuristic
developments. J Cardiol Cardiovasc Ther 3: 555603.
4.
Contreras-Zentella ML, Hernández-Muñoz R (2016) Is
liver enzyme release really associated with cell necrosis induced by oxidant
stress? Oxid Med Cell Longev 2016: 3529149.
5.
Schmidt E, Schmidt FW (1970) Aspects of enzyme
diagnosis. Med Welt 21: 805-816.
6.
Frederiks WM, Vogels IM, Fronik GM (1984) Plasma
ornithine carbamyl transferase level as an indicator of ischemic injury of rat
liver. Cell Biochem Funct 2: 217-220.
7.
Boots AW, Haenen GR, Bast A (2003) Oxidant
metabolism in chronic obstructive pulmonary disease. Eur Respir J 46: 14-27.
8.
Romieu I (2005) Nutrition and lung health. Int J
Tuberc Lung Dis 9: 362-374.
9.
Kelly FJ (2005) Vitamins and respiratory disease:
Antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc 64:
510-526.
10.
Fischer AG, Ullsperger M (2017) An update on the
role of serotonin and its interplay with dopamine for reward. Front Hum
Neurosci 11: 484.
11.
Anonymous (2006) A sensible 10-year plan for mental
health. Lancet 367: 86.
12.
Ryan-Harshman M, Aldoori W (2005) Health benefits
of selected minerals. Can Fam Physician 51: 673-675.
13.
Rayman MP (2000) The importance of selenium to
human health. Lancet 356: 233-241.
14.
Beard JL, Connor JR (2003) Iron status and neural
functioning. Ann Rev Nutr 23: 41-58.
15.
Coleman CI, Hebert JH, Reddy P (2003) The effects
of Panax ginseng on quality of life. J Clin Pharm Ther 28: 5-15.
16.
Das L, Bhaumik E, Raychaudhuri U, Chakraborty R
(2011) Role of nutraceuticals in human health. J Food Sci Technol 49:173-183.
17.
Lutgendorf SK, Mullen-Houser E, Russell D, Degeest
K, Jacobson G et al. (2010) Preservation of immune function in cervical cancer
patients during chemoradiation using a novel integrative approach. Brain Behav
Immun 24: 1231-1240.
18.
Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M
et al. (1996) Massage therapy is associated with enhancement of the immune
system's cytotoxic capacity. Int J Neurosci 84: 205-217.
19.
Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R
et al. (2015) Clinical studies of biofield therapies: Summary, methodological
challenges and recommendations. Glob Adv Health Med 4: 58-66.
20.
Rubik B (2002) The biofield hypothesis: Its
biophysical basis and role in medicine. J Altern Complement Med 8: 703-717.
21.
Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S
(2015) The potential impact of biofield treatment on human brain tumor cells: A
time-lapse video microscopy. J Integr Oncol 4: 141.
22.
Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S
(2015) In vitro evaluation of biofield treatment on cancer biomarkers
involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7:
253-257.
23.
Trivedi MK, Tallapragada RM (2008) A transcendental
to changing metal powder characteristics. Met Powder Rep 63: 22-28, 31.
24.
Trivedi MK, Nayak G, Patil S, Tallapragada RM,
Latiyal O (2015) Studies of the atomic and crystalline characteristics of
ceramic oxide nanopowders after bio field treatment. Ind Eng Manage 4: 161.
25.
Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S
et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter
braakii: A urinary pathogen. J Clin Med Genom 3: 129.
26.
Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S
(2015) An impact of biofield treatment: Antimycobacterial susceptibility
potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5: 189.
27.
Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal
SC et al. (2015) Morphological characterization, quality, yield and DNA
fingerprinting of biofield energy treated alphonso mango (Mangifera indica
L.). Journal of Food and Nutrition Sciences 3: 245-250.
28.
Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal
SC et al. (2015) Evaluation of biochemical marker – Glutathione and DNA
fingerprinting of biofield energy treated Oryza sativa. Am J Biosci 3: 243-248.
29.
Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd
WD et al. (2017) A Systematic study of the biofield energy healing treatment on
physicochemical, thermal, structural and behavioral properties of magnesium
gluconate. Int J Bioorg Chem 2: 135-145.
30.
Parulkar VR, Trivedi MK, Branton A, Trivedi D,
Nayak G et al. (2018) Improved metabolism of vitamin D3 in human osteoblasts
cells after biofield energy healing treatment. Am J Lab Med 3: 11-19.
31.
Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S
(2015) Phenotypic and biotypic characterization of Klebsiella oxytoca:
An impact of biofield treatment. J Microb Biochem Technol 7: 203-206.
32.
Nayak G, Altekar N (2015) Effect of biofield
treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
33.
Branton A, Jana S (2017) The influence of energy of
consciousness healing treatment on low bioavailable resveratrol in male Sprague
Dawley rats. Int J Clin Dev Anatomy 3: 9-15.
34.
Branton A, Jana S (2017) The use of novel and
unique biofield energy healing treatment for the improvement of poorly
bioavailable compound, berberine in male Sprague Dawley rats. Am J Clin Exp Med
5: 138-144.
35.
Branton A, Jana S (2017) Effect of The biofield
energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3
[25(OH)D3] in rats after a single oral dose of vitamin D3. Am J Pharmacol
Phytother 2: 11-18.
36.
Parulkar VR, Trivedi MK, Branton A, Trivedi D,
Nayak G et al. (2017) The use of consciousness energy healing based
herbomineral formulation for skin anti-aging strategies. J Food Nutr Sci 5:
96-106.
37.
Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G
et al. (2017) Consciousness energy healing treatment based herbomineral
formulation: A safe and effective approach for skin health. Am J Pharmacol
Phytother 2: 1-10.
38.
Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G
et al. (2018) Influence of biofield treated vitamin D3 on proliferation,
differentiation and maturation of bone-related parameters in MG-63 cell-line.
Int J Biomed Eng Clin Sci 4: 6-14.
39.
Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G et
al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone
mineralization in human bone osteosarcoma cells (MG-63). Int J Nutr Food Sci 7:
30-38.
40.
Stutheit ME, Trivedi K, Branton A, Trivedi D, Nayak
G et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on
bone health using osteoblasts cells. Am J Life Sci 6: 13-21.
41.
Alía M, Ramos S, Mateos R, Bravo L, Goya L (2005)
Response of the antioxidant defense system to tert-butyl hydroperoxide and
hydrogen peroxide in a human hepatoma cell line (HepG2). J Biochem Mol Toxicol
19: 119-128.
42.
Atkins GJ, Findlay DM, Anderson PH, Morris HA
(2011) Vitamin D. 3rd Edn. Volume I Chapter 23 – Target Genes: Bone
Proteins, pp: 411-424.
43.
Emami A, Larsson A, Petrén-Mallmin M, Larsson S
(1999) Serum bone markers after intramedullary fixed tibial fractures. Clin
Orthop Relat Res 368: 220-229.
44.
Komnenou A, Karayannopoulou M, Polizopoulou ZS,
Constantinidis TC, Dessiris A (2005) Correlation of serum alkaline phosphatase
activity with the healing process of long bone fractures in dogs. Vet Clin
Pathol 34: 35-38.
45.
Burgner JW, Ray WJ (1984) On the origin of the
lactate dehydrogenase induced rate effect. Biochemistry 23: 3636-3648.
46.
Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ
(2015) The regulation and function of lactate dehydrogenase A: Therapeutic
potential in brain tumor. Brain Pathol 26: 3-17.
47.
Kopperschläger G, Kirchberger J (1996) Methods for
the separation of lactate dehydrogenases and clinical significance of the
enzyme. J Chromatogr B Biomed Appl 684: 25-49.
48.
Pratt DS, Kaplan MM (2000) Evaluation of abnormal
liver-enzyme results in asymptomatic patients. N Engl J Med 342: 1266-1271.
49.
Mathiesen U, Franzen L, Fryden A, Foberg U, Bodemar
G (1999) The clinical significance of slightly to moderately increased liver
transaminase values in asymptomatic patients. Scand J Gastroenterol 34: 85-91.
50.
Sahiner UM, Birben E, Erzurum S, Sackesen C,
Kalayci O (2011) Oxidative stress in asthma. World Allerg Organ J 4: 151-158.
51.
Younus H (2018) Therapeutic potentials of
superoxide dismutase. Int J Health Sci (Qassim) 12: 88-93.
52.
Martin SL, Power A, Boyle Y, Anderson IM,
Silverdale MA, Jones AKP (2017) 5-HT modulation of pain perception in humans.
Psychopharmacology (Berl) 234: 2929-2939.
53.
da Cunha-Bang S, Mc Mahon B, Fisher PM, Jensen PS,
Svarer C, Knudsen GM (2016) High trait aggression in men is associated with low
5-HT levels, as indexed by 5-HT4 receptor binding. Soc Cogn Affect Neurosci 11:
548-555.
54.
Lee SM, Meyer MB, Benkusky NA, O'Brien CA, Pike JW
(2018) The impact of VDR expression and regulation in vivo. J Steroid
Biochem Mol Biol 177: 36-45.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Astronomy and Space Research
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)