655
Views & Citations10
Likes & Shares
Efflux pumps are omnipresent in almost all types of
cells. They participate in the transportation of a wide range of important
molecules across the cell membrane. They play primordial roles in
detoxification of the cell by disposing off unwanted materials. Since tumor
cells emerge from normal cells as a result of mutation, they carry with them
the genes coding for the efflux pumps. These efflux pumps are overexpressed by
cancer cells. They defend the cancer cells against chemotherapy by actively
pumping out the drug molecules that have incurred into the cytoplasm. This
leads to drug resistance, which is the major cause for low efficiency of most
of the chemotherapeutic drugs. Although many efflux pump inhibitors have been
developed, none of them has been clinically approved because of their lack in
specificity, which causes them to incur into the normal cells leading to many
adverse effects. Moreover, with time, the cancer cells develop resistance
against these inhibitors. RNAi mediated gene silencing proved to be effective
in silencing the MDR genes under in vitro
conditions. However, they prove to be inefficient under in vivo trials due to the lack of a proper transport vector that
will be able to transport the pre-interfering RNAs specifically to the tumor
site while keeping the normal cells intact. Many oncolytic viruses have been
identified and genetically engineered to specifically infect a wide range of
tumor cells. This article proposes the use of genetically modified oncolytic
viruses as transport vectors for the pre-interfering RNAs to solve the above
problems. The strategies proposed in this article can be employed to specifically
target the MDR genes present in cancer cells, while keeping the normal cells
untouched and can be used adjunct to chemotherapy to make it efficacious.
Keywords: RNAi,
Efflux pumps, Chemotherapy
BACKGROUND
Abnormal cell division as a result of
mutation leads to cancer. Today, it is one of the leading causes of mortality
globally, leading to 8.8 million deaths every year [1]. In a global survey
carried out in 2015, 90.5 million people were said to suffer from cancer, which
increases by 14.1 million every year and this rate is expected to surpass 20
million by the end of 2025 [2,3]. The different treatments involved in cancer
include chemotherapy, immune therapy, radiation therapy and surgery [4,5].
However, the most widely used therapies against cancer are chemotherapy and
radiation therapy [6]. It is evident that with the passage of time the cancer
cells become more virulent and their resistance to chemotherapy increase [7].
The increase in resistance of the cancer cells against chemotherapeutic drugs
can be attributed to the overexpression of Multidrug Resistance efflux pumps.
Since the tumor cells emerge from normal
cells as a result of mutation, they carry with them the genes coding for the
Efflux pumps. An over-expression of efflux pump proteins is evident in a wide
range of cancer cells [25-31]. The cancer cells overexpress efflux pumps, which
defend them against chemotherapeutic drugs by actively pumping them out of the
cell, thus reducing their intracellular concentration. The efflux pumps belong
to the ABC (ATP binding cassette) transporter family which utilizes the energy
obtained from ATP hydrolysis for the transport of different molecules. The most
extensively expressed members of the ABC family involved in Multidrug
Resistance are transport proteins ABCB1, ABCC1 and ABG2 [32].
In addition, some of the efflux transporter
proteins also act as MHCs (major histocompatibility complex) and play
regulatory roles in cell signaling [33]. Several members of the ABC transporter
family have been depicted to be involved in evading apoptosis and inducing
proliferation of the tumor cells. For example, transport proteins ABCB1 and
ABCC1 play anti-apoptotic roles in tumor cells by delaying response to
apoptotic signals [34-36]. Similarly, transport proteins ABCC1, ABCC4 and ABCG2
bolster proliferation in cancer cells [37-39].
NEED FOR A TRANSPORT
VEHICLE
Over the years many researches have been
carried out in developing drugs targeting the efflux pumps, to be used adjunct
to chemotherapy. Many inhibitors have been developed against the multidrug
resistance efflux pumps such as quinine, quinidine, verapamil, cyclosporin,
PSC-833, MS-209 and others [32]. However, they proved to be disappointments with
very limited rates of clinical success [32,40-46]. Most of them cause drug
related adverse effects due to the lack of specificity, by also damaging the
efflux pumps that are present on normal cells [47].
Efforts to silence the MDR genes have already
been carried out using different molecular biology tools such as antisense
therapy, ribozyme therapy and RNA interference [48-50]. RNA interference via
Small interfering RNA and short hairpin RNA designed to inactivate the MDR
genes proved to be quite efficient in abating drug resistance in cancer cells
[51,52]. Although RNA interference is an efficient technique for gene silencing
under in vitro conditions, it fails
to meet the expectations when subjected to in
vivo due to many factors such as the low bioavailability of the RNA
molecules at the target site, as most of them get excreted from the body
through urine, many are destroyed due to nuclease activity and the others fail
to enter the cell due to their negative charge and large size [53-55]. In
addition, many molecules that manage to successfully pass through the membrane
through endocytosis get degraded inside the endosome before reaching the
cytoplasm [56]. Moreover, using a vector that cannot unload the pre-interfering
RNA molecules specifically at the tumor site may abate the expression of efflux
pumps in normal cells leading to many adverse effects in the body. The above
factors mark the need for a vector that can safely transport the RNA molecules
to their target site [51,57-60]. Using oncolytic viruses as transport systems
for the pre-interfering RNA molecules can solve these problems.
EMPLOYING ONCOLYTIC
VIRUSES TO SILENCE THE MULTIDRUG RESISTANCE GENES VIA RNA INTERFERENCE
Tumor cells are formed as a result of
mutation in normal cells which gives them the ability to evade immune
responses, to proliferate limitlessly and to evade apoptosis. This makes them
an interesting target for viruses to grow in. Some oncolytic viruses exist
naturally. However, most of them are genetically engineered to make them specific
to cancer cell. Some extensively employed oncolytic viruses are Adenovirus,
Chicken anemia virus, Parvovirus, Herpes Simplex virus and Newcastle disease
virus [61].
The upcoming topics propose the use of
Oncolytic viruses in silencing the MDR genes:
Using viral shells
as transport vehicles for pre-interfering RNAs
Certain protein receptors are overexpressed
on the surfaces of tumor cells. They serve as the entry ligands for many
viruses. For example, the intra-cellular adhesion molecule-1 (ICAM-1) and decay
accelerating factor (DAF) which serve as entry receptors for coxsackievirus A21
are over expressed by certain cancer cells [62,63]. Similarly, human ovarian
cancer cells overexpress α2β1 integrin which serves as the entry receptor for
echovirus type 1 [64]. The viruses whose entry receptors are over expressed by
cancer cells can be exploited for selectively targeting the tumor cells [61].
This targeting strategy is called transductional targeting.
In case of enveloped viruses, the viral shell
comprises of the envelope and the capsid, whereas, in case of non-enveloped
viruses it comprises only of the viral capsid. Since, the surface proteins
present on viral shells are involved in the transductional targeting of tumor
cells; they can be used as transport vehicles for safely transferring the
pre-interfering RNAs to the cancer cell, without causing them to intervene into
normal cells.
Only the viruses whose entry receptors are
overexpressed by the cancer cells can be chosen for this strategy. The part of
their genome coding for the capsid, envelope and other associated proteins
should be isolated, amplified and expressed in a protein expression system. The
proteins formed will then self-assemble to form functional viral shells lacking
any genetic material [65-71]. Baculo virus expression system is the most widely
used protein expression system for this purpose [72]. At present, many
virus-like particles lacking genetic material, that mimic the original virus
have being developed using this method, to be used as vaccines to stimulate
humoral and cellular immunity [65-80].
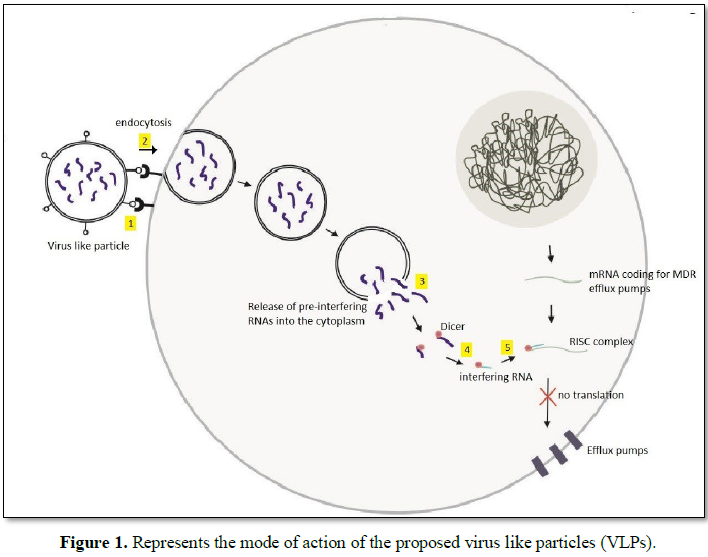
Step 1 depicts the attachment of the VLP to
the cancer cell as a result of ligand-receptor binding. This interaction causes
the uptake of the VLP through endocytosis as depicted in step 2. On entering
the cancer cell, the VLP will lyse, freeing the RNA molecules into the
cytoplasm as in step 3. In step 4 the RNA molecules are cleaved by dicer to
form short interfering RNAs. These small interfering RNAs separate into guide
RNA and passenger RNA. The guide RNA combines with argonaute and other
associated proteins to form the RISC complex which then binds with the target
mRNA, leading to its inactivation as depicted in step 5.
The virus like particles on being injected
will get dispersed throughout the body though blood. On reaching the tumor
cells, the viral receptors will interact with the receptors overexpressed on
the surface of tumor cells leading to its attachment [83]. The clustering of
receptors will give rise to a signaling cascade which will initiate the uptake
of the virus like particle through endocytosis or macro pinocytosis [83]. On
entering the cell, the viral capsids will dissolve, releasing the
pre-interfering RNA molecules into the cytoplasm. These exogenous dsRNAs or
shRNAs will activate the ribonuclease protein Dicer present in the cytoplasm, which
will cleave the double stranded or hairpin RNAs to form short stretches of
double stranded RNA about 25 bp long [84]. These short double stranded RNAs,
also called short interfering RNA will then unwind, giving rise to two short
single stranded RNAs called passenger and guide RNA, respectively. The
passenger RNA will degenerate, whereas the guide RNA will get loaded up on an
Argonaute protein which will then bind with the target mRNA to form the
RNA-induced silencing complex (RISC) [85]. The complex formed will block the
mRNA from getting translated [86]. The RISC complex can also induce the
Argonaute protein “slicer” to cleave the target mRNA and in this way the
expression of the target gene can be attenuated to a great extent [87].
Using oncolytic viruses
to carry out DNA vector based RNAi
Although the previously described strategy
sounds promising, it can be surmised to carry some drawbacks. Firstly, the
targeting strategy is limited to transductional targeting and only a few types
of cancer cells have been known till date, to overexpress viral entry
receptors. Secondly, the proposed virus like particles are not capable of
self-replication, they need to be synthesized manually which may lead to an
increase in their production cost.
Creating a self-replicable genetically
modified DNA virus or a retrovirus, that will be able to replicate inside a
wide range of cancer cells and will be to produce pre-interfering RNAs
naturally through transcription can help to counter the problems associated
with the previous strategy.
Firstly, the virus should be made
nonpathogenic by attenuating its harmful genes. Then, based on the requirement,
the virus should be made cancer cell specific by genetically modifying it in
accordance with any of the following strategies:
Proapoptotic
signaling: Viral
intrusion into a normal cell can trigger apoptotic signaling cascade which can
bring about many morphological and biochemical changes leading to cell death,
thus preventing viral replication [88,89]. Some viruses can synthesize certain
proteins which can inhibit apoptotic signaling thus providing them enough time
to replicate [90]. However, cancer cells generally have a defective apoptotic
pathway [91]. If the viral genes coding for the anti-apoptotic proteins are
mutated then the resulting virus will fail to replicate inside normal cells due
to its inability to inhibit the virus triggered apoptotic pathway [92].
However, it will be able to grow inside cancer cells. For example, Onyx-15, a genetically modified
adenovirus with attenuated Eb1 gene can grow selectively in p53 deficient
cancer cells [93].
Transcriptional
targeting: There
are certain essential viral genes that are necessary for viral replication.
Placing these genes under the regulation of tumor specific promoter can make
the virus tumor specific by seizing its ability to replicate under non-tumor
environment [94]. Hence the viruses will only be able express its vital genes
and replicate it inside tumor cells.
Translation
targeting: A
virus infected cell produces Type I IFN (interferon) which ceases protein
synthesis in its neighboring cells thus making them unfit for viral infection
[95]. Engineering viruses to initiate a more potent IFN response in normal
cells will prevent the viruses from spreading into its surrounding cells [90].
However, as the cancer cells have a defective IFN pathway the genetically
modified viruses will fail to initiate an IFN response in cancer cells thus
permitting viral replication inside them. Some viruses can block IFN signaling
by encoding certain proteins that can inhibit the IFN signaling pathway [96].
Mutating the IFN inhibiting genes can prevent the viruses from replicating in
normal cells [90]. On the other hand, the virus will be able to replicate in
cancer cells as they have a defective IFN signaling pathway which cannot
suppress viral replication [90].
The gene suppression strategy used here would
be based on DNA vector based RNAi technology [88]. After the virus has been
made cancer cell specific, the next step involves constructing a gene which on
transcription will give rise to shRNAs, complementary to the target gene. The
gene should consist of a promoter followed by two complementary sequences
separated by a short non-homologous spacer DNA [88]. The two complementary
sequences should be made in accordance with the sequence of the mRNA to be
silenced. This gene should then be integrated into the genome of the oncolytic
virus.
On being subjected to in vivo trials, the genetically modified virus will fail to
proliferate inside normal cells. However, on infecting a cancer cell it will be
able to replicate itself as well as transcribe the shRNAs which will then form
RISC complex with the target mRNA and degrade it using the same procedure that
was stated earlier. This will diminish the expression of Multidrug Resistance
efflux pumps in the viral infected tumor cells.
The use of genetically engineered DNA viruses
and retroviruses offers a wide range of targeting strategies to be employed to
target a broad range of cancer cells. Since the viruses are self-replicable, it
will be easy to clone them in cancer cell cultures which will also reduce their
production cost.
CONSEQUENCES
The strategies proposed in this article can
be used to abate the expression of Multidrug Resistance efflux pumps in cancer
cells. Using the proposed strategy, adjunct to chemotherapy will make it more
effective. In the absence of efflux pumps, the cancer cells will fail to defend
themselves against the anti-cancer drugs. Hence the chemotherapeutic drugs will
be able to eliminate the cancer cells without much difficulty. This strategy
can also be used to target other important genes expressed in cancer cells that
are also common to normal cells.
CONFLICT OF INTEREST
1. GBD
(2016) Mortality and causes of death collaborators, global, regional and
national life expectancy, all-cause mortality and cause-specific mortality for
249 causes of death, 1980-2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet 388: 1459-1544.
2. GBD
(2016) Disease and injury incidence and prevalence collaborators, global,
regional and national incidence, prevalence and years lived with disability for
310 diseases and injuries, 1990-2015: A systematic analysis for the Global
Burden of Disease Study 2015. Lancet 388: 1545-1602.
3. World
Health Organization (2014) World cancer report 2014. International Agency for
Research on Cancer, p: 64.
4. World
Health Organization (2018) Cancer.
5. National
Cancer Institute (2019) Targeted cancer therapies.
6. National
Cancer Institute (2015) Cancer treatment. Available at: http://www.cancer.gov/about-cancer/treatment
7. Cancer
Research UK (2017) Why some cancers come back?
8. Juliano
RL, Ling VA (1976) Surface glycoprotein modulating drug permeability in Chinese
hamster ovary cell mutants. Biochimica et Biophysica Acta (BBA) – Bio membranes
455: 152-162.
9. Johnstone
RW, Ruefli AA, Smyth MJ (2000) Multiple physiological functions for multidrug
transporter P-glycoprotein? Trends Biochem Sci 25: 1-6.
10. Demeule
M, Régina A, Jodoin J, Laplante A, Dagenais C, et al. (2002) Drug transport to
the brain: Key roles for the efflux pump P-glycoprotein in the blood-brain
barrier. Vascul Pharmacol 38: 339-348.
11. Bambeke
V, Michot JM, Tulkens P (2003) Antibiotic efflux pumps in eukaryotic cells:
Occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics
and toxicodynamics. J Antimicrob Chemother 51: 1067-1077.
12. Albrecht
C, Viturro E (2006) The ABCA subfamily—gene and protein structures, functions
and associated hereditary diseases. Pflugers Arch 453: 581-589.
13. Takahashi
K, Kimura Y, Nagata Y (2005) ABC proteins: Key molecules for lipid homeostasis.
Med Mol Morphol 38: 2-12.
14. Dean
M, Fojo T, Bates S (2005) Tumor stem cells and drug resistance. Nature Rev
Cancer 5: 275-284.
15. Cormet-Boyaka
E, Huneau JF, Mordrelle A, Boyaka PN, Carbon C, et al. (1998) Secretion of
sparfloxacin from the human intestinal Caco-2 cell line is altered by
P-glycoprotein inhibitors. Antimicrob Agents Chemother 42: 2607-2611.
16. Zhao
YL, Cai SH, Wang L, Kitaichi K, Tatsumi Y, et al. (2002) Possible involvement
of P-glycoprotein in the biliary excretion of grepafloxacin. Clin Exp Pharmacol
Physiol 29: 167-172.
17. Ito
T, Yano I, Tanaka K, Ichi Inui K (1997) Transport of quinolone antibacterial
drugs by human P-glycoprotein expressed in a kidney epithelial cell line,
LLC-PK1. J Pharmacol Exp Ther 282: 955-960.
18. van
Helvoort A, Smith AJ, Sprong H, Schinkel AH, Borst P, et al. (1996) MDR1
P-glycoprotein is a lipid translocase of broad specificity, while MDR 3
P-glycoprotein specifically translocates phosphatidylcholine. Cell 87: 507-517.
19. Luker
GD, Nilsson KR, Covey DF, Piwnica-Worms D (1999) Multidrug resistance (MDR1)
P-glycoprotein enhances esterification of plasma membrane cholesterol. J Biol
Chem 274: 6979-6991.
20. Hardy
SP, Good fellow HR, Valverde MA, Gill DR, Sepulveda V, et al. (1995) Protein
kinase C-mediated phosphorylation of the human multidrug resistance
P-glycoprotein regulates cell volume-activated chloride channels. EMBO J 14:
68-75.
21. Drach
J, Gsur A, Hamilton G, Zhao S, Angerler J, et al. (1996) Involvement of
P-glycoprotein in the trans membrane transport of interleukin-2 (IL-2), IL-4
and interferon-gamma in normal human T-lymphocytes. Blood 88: 1747-1754.
22. Sharma
RC, Inoue S, Roitelman J, Schimke RT, Simoni RD (1992) Peptide transport by the
multidrug resistance pump. J Biol Chem 267: 5731-5734.
23. Amin
ML (2013) P-glycoprotein inhibition for optimal drug delivery. Drug Target
Insights 7: 27-34.
24. Smyth
MJ, Trapani JA (1995) Granzymes: Exogenous proteinases that induce target cell
apoptosis. Immunol Today 16: 202-206.
25. Zochbauer-Muller
S, Filipits M, Rudas M (2001) P-glycoprotein and MRP1 expression in axillary
lymph node metastases of breast cancer patients. Anticancer Res 21: 119-124.
26. Filipits
M, Suchomel R, Dekan G, Haider K, Valdimarsson G, et al. (1996) MRP and MDR1
gene expression in primary breast carcinomas. Clin Cancer Res 2: 1231-1237.
27. König
J, Hartel M, Nies AT, Martignoni ME, Guo J, et al. (2005) Expression and
localization of human multidrug resistance protein (ABCC) family members in
pancreatic carcinoma. Int J Cancer 115: 359-367.
28. Steinbach
D, Gillet J, Sauerbrey A, Gruhn B, Dawczynski K, et al. (2006) ABCA3 as a
possible cause of drug resistance in childhood acute myeloid leukemia. Clin
Cancer Res 12: 4357-4363.
29. Weinstein
RS, Jakate S, Dominguez JM, Lebovitz MD, Koukoulis GK, et al. (1991) Relationship
of the expression of the multidrug resistance gene product (P-glycoprotein) in
human colon carcinoma to local tumor aggressiveness and lymph node metastasis.
Cancer Res 51: 2720-2726.
30. Oda
Y, Saito T, Tateishi N, Ohishi Y, Tamiya S, et al. (2005) ATP-binding cassette
superfamily transporter gene expression in human soft tissue sarcomas. Int J
Cancer 114: 854-862.
31. Ohtsuki
S, Kamoi M, Watanabe Y, Suzuki H, Hori S, et al. (2007) Correlation of
induction of ATP binding cassette transporter A5 (ABCA5) and ABCB1 mRNAs with
differentiation state of human colon tumor. Biol Pharm Bull 30: 1144-1146.
32. Szakács
G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting
multidrug resistance in cancer. Nat Rev Drug Discov 5: 219-234.
33. Fletcher
JI, Haber M, Henderson MJ, Norris MD (2010) ABC transporters in cancer: More
than just drug efflux pumps. Nat Rev Cancer 10: 147-156.
34. Robinson
LJ, Roberts WK, Ling TT, Lamming T, Sternberg SS, et al. (1997) Human MDR 1
protein overexpression delays the apoptotic cascade in Chinese hamster ovary
fibroblasts. Biochemistry 36: 11169-11178.
35. Smyth
MJ, Krasovskis E, Sutton VR, Johnstone RV (1998) The drug efflux protein,
P-glycoprotein, additionally protects drug-resistant tumor cells from multiple
forms of caspase-dependent apoptosis. Proc Natl Acad Sci U S A 95: 7024-7029.
36. Peaston
AE, Gardaneh M, Franco AV, Hocker JE, Murphy KM, et al. (2001) MRP1 gene
expression level regulates the death and differentiation response of
neuroblastoma cells. Br J Cancer 85: 1564-1571.
37. Kuss
B, Corbo M, Lau WM, Fennell DA, Dean NM, et al. (2002) In vitro and in vivo down
regulation of MRP1 by antisense oligonucleotides: A potential role in
neuroblastoma therapy. Int J Cancer 98: 128-133.
38. Sassi
Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, et al. (2008) Multidrug
resistance-associated protein 4 regulates cAMP-dependent signaling pathways and
controls human and rat SMC proliferation. J Clin Invest 118: 2747-2757.
39. Bhattacharya
S, Das A, Mallya K, Ahmad I (2007) Maintenance of retinal stem cells by Abcg2
is regulated by notch signaling. J Cell Sci 120: 2652-2662.
40. Wishart
GC, Bissett D, Paul J, Jodrell D, Harnett A, et al. (1994) Quinidine as a
resistance modulator of epirubicin in advanced breast cancer: Mature results of
a placebo-controlled randomized trial. J Clin Oncol 12: 1771-1777.
41. Solary
E, Witz B, Caillot D, Moreau P, Desablens B, et al. (1996) Combination of
quinine as a potential reversing agent with mitoxantrone and cytarabine for the
treatment of acute leukemias: A randomized multicenter study. Blood 88:
1198-1205.
42. Milroy
R (1993) A randomised clinical study of verapamil in addition to combination
chemotherapy in small cell lung cancer. West of Scotland Lung Cancer Research
Group and the Aberdeen Oncology Group. Br J Cancer 68: 813-818.
43. Dalton
WS, Crowley JJ, Salmon SS, Grogan TM, Laufman LR, et al. (1995) A phase III
randomized study of oral verapamil as a chemosensitizer to reverse drug
resistance in patients with refractory myeloma. A Southwest Oncology Group
study. Cancer 75: 815-820.
44. Yin
JL, Wheatley K, Rees JK, Burnett AK (2001) Comparison of ‘sequential’ versus
’standard’ chemotherapy as re-induction treatment, with or without
cyclosporine, in refractory/relapsed acute myeloid leukemia (AML): Results of
the UK Medical Research Council AML-R trial. Br J Hematol 113: 713-726.
45. van
der Holt B, Löwenberg B, Burnett AK, Knauf WU, Shepherd J, et al. (2005) The
value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and
cytarabine in the treatment of elderly patients with previously untreated acute
myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood 106:
2646-2654.
46. Sonneveld
P, Suciu S, Weijermans P, Beksac M, Neuwirtova R, et al. (2001) Cyclosporin A
combined with vincristine; doxorubicin and dexamethasone (VAD) compared with
VAD alone in patients with advanced refractory multiple myeloma: An EORTC-HOVON
randomized phase III study (06914). Br J Hematol 115: 895-902.
47. Relling
MV (1996) Are the major effects of P-glycoprotein modulators due to altered
pharmacokinetics of anticancer drugs? Ther Drug Monit 18: 350-356.
48. Ramachandran
C, Wellham LL (2003) Effect of MDR1 phosphorothioate antisense
oligodeoxynucleotides in multidrug-resistant human tumor cell lines and
xenografts. Anticancer Res 23: 2681-2690.
49. Stuart
DD, Kao GY, Allen TM (2000) A novel, long-circulating and functional liposomal
formulation of antisense oligodeoxynucleotides targeted against MDR1. Cancer
Gene Ther 7: 466-475.
50. Masuda
Y, Kobayashi H, Holland JF, Ohnuma T (1998) Reversal of multidrug resistance by
a liposome-MDR1 ribozyme complex. Cancer Chemother Pharmacol 42: 9-16.
51. Xu H,
Hong FZ, Li S, Zhang P, Zhu L (2012) Short hairpin RNA-mediated MDR1 gene
silencing increases apoptosis of human ovarian cancer cell line A2780/Taxol.
Chin J Cancer Res 24: 138-142.
52. Yague
E, Higgins CF, Raguz S (2004) Complete reversal of multidrug resistance by
stable expression of small interfering RNAs targeting MDR1. Gene Ther 11:
1170-1174.
53. Guo
P, Coban O, Snead NM, Trebley J, Hoeprich S, et al. (2010) Engineering RNA for
Targeted siRNA delivery and medical application. Adv Drug Deliv Rev 62:
650-666.
54. Alexis
F, Pridgen E, Molnar LK, Farokhzad OC (2008) Factors affecting the clearance
and bio distribution of polymeric nanoparticles. Mol Pharm 5: 505-515.
55. Aagaard
L, Rossi JJ (2007) RNAi therapeutics: Principles, prospects and challenges. Adv
Drug Deliv Rev 59: 75-86.
56. Zhang
B, Mallapragada S (2011) The mechanism of selective transfection mediated by
penta block copolymers; Part II: Nuclear entry and endosomal escape. Acta
Biomater 7: 1580-1587.
57. Deng
Y, Wang CC, Choy KW, Du Q, Chen J, et al. (2014) Therapeutic potentials of gene
silencing by RNA interference: Principles, challenges and new strategies. Gene
538: 217-227.
58. Xu D,
Kang H, Fisher M, Juliano RL (2004) Strategies for inhibition of MDR1 gene
expression. Mol Pharmacol 66: 268-275.
59. Pichler
A, Zelcer N, Prior JL, Kuil AJ, Piwnica-Worms D (2005) In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein.
Clin Cancer Res 11: 4487-4494.
60. Stevenson
M (2004) Therapeutic potential of RNA interference. N Engl J Med 351:
1772-1777.
61. Singh
PK, Doley J, Kumar GR, Sahoo AP, Tiwari AK (2012) Oncolytic viruses and their
specific targeting to tumor cells. Indian J Med Res 136: 571-584.
62. Palmer
DH, Young L, Mautner V (2006) Cancer gene-therapy: Clinical trials. Trends
Biotechnol 24: 76-82.
63. Au
GG, Lindberg AM, Barry RD, Shafren DR (2005) Oncolysis of vascular malignant
human melanoma tumors by Coxsackievirus A21. Int J Oncol 26: 1471-1476.
64. Shafren
DR, Sylvester D, Johansson ES, Campbell IG, Barry RD (2005) Oncolysis of human
ovarian cancers by echovirus type 1. Int J Cancer 115: 320-328.
65. Jiang
X, Wang M, Graham DY, Estes MK (1992) Expression, self-assembly and
antigenicity of the Norwalk virus capsid protein. J Virol 66: 6527-6532.
66. Laurent
S, Vautherot JF, Madelaine MF, Le Gall G, Rasschaert D (1994) Recombinant
rabbit hemorrhagic disease virus capsid protein expressed in baculovirus
self-assembles into virus like particles and induces protection. J Virol 68:
6794-6798.
67. Hale
AD, Crawford SE, Ciarlet M, Green J, Gallimore C (1999) Expression and
self-assembly of Grimsby virus: Antigenic distinction from Norwalk and Mexico
viruses. Clin Vaccin Immunol 6: 142-145.
68. Li
TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, et al. (1997) Expression and
self-assembly of empty virus-like particles of hepatitis. J Virol 71:
7207-7213.
69. Martinez
C, Dalsgaard K, de Turiso JL, Cortés E, Vela C, Casal JI (1992) Production of
porcine parvovirus empty capsids with high immunogenic activity. Vaccine 10:
684-690.
70. Brown
CS, Lent JV, Vlak JM, Spaan WJ (1991) Assembly of empty capsids by using
baculovirus recombinants expressing human parvovirus B19 structural proteins. J
Virol 65: 2702-2706.
71. Christensen
J, Alexandersen S, Bloch B, Aasted B, Uttenthal A (1994) Production of mink
enteritis parvovirus empty capsids by expression in a baculovirus vector
system: A recombinant vaccine for mink enteritis parvovirus in mink. J Gen
Virol 75: 149-155.
71.
72. Noad
R, Roy P (2003) Virus-like particles as immunogens. Trends Microbiol 11:
438-444.
73. Yao
Q, Kuhlmann FM, Eller R, Compans RW, Chen C (2000) Production and
characterization of simian/human immunodeficiency virus-like particles. AIDS
Res Hum Retroviruses 16: 227-236.
74. Betenbaugh
M, Yu M, Kuehl K, White J, Pennock D, et al. (1995) Nucleocapsid and virus-like
particles assemble in cells infected with recombinant baculoviruses or vaccinia
viruses expressing the M and the S segments of Hantaan virus, the M and the S
segments of Hantaan virus. Virus Res 38: 111-124.
75. Yamshchikov
GV, Ritter GD, Vey M, Compans RW (1995) Assembly of SIV virus-like particles
containing envelope proteins using a baculovirus expression system. Virology
214: 50-58.
76. Baumert
TF, Ito S, Wong DT, Liang TJ (1998) Hepatitis C virus structural proteins
assemble into virus-like particles in insect cells. J Virol 72: 3827-3836.
77. Latham
T, Galarza JM (2001) Formation of wild-type and chimeric influenza virus-like
particles following simultaneous expression of only four structural proteins. J
Virol 75: 6154-6165.
78. Sabara
M, Parker M, Aha P, Cosco C, Gibbons E, et al. (1991) Assembly of
double-shelled rotavirus like particles by simultaneous expression of
recombinant VP6 and VP7 proteins. J Virol 65: 6994-6997.
79. Jiang
B, Estes MK, Barone C, Barniak V, O'Neal CM, et al. (1999) Heterotypic
protection from rotavirus infection in mice vaccinated with virus-like
particles. Vaccine 17: 1005-1013.
80. French
TJ, Roy P (1990) Synthesis of bluetongue virus (BTV) core like particles by a
recombinant baculovirus expressing the two major structural core proteins of
BTV. J Virol 64: 1530-1536.
81. Sohail
M, Doran G, Riedemann J, Macaulay V, Southern EM (2003) A simple and
cost-effective method for producing small interfering RNAs with high efficacy.
Nucleic Acids Res 31: e38.
82. Cadena-Nava
RD, Comas-Garcia M, Garmann RF, Rao ALN, Knobler CM, et al. (2011)
Self-assembly of viral capsid protein and RNA molecules of different sizes:
requirement for a specific high protein/RNA mass ratio. J Virol 86: 3318-3326.
83. Cossart
P, Helenius A (2014) Endocytosis of viruses and bacteria. Cold Spring Harb
Perspect Biol 6: a016972.
84. Bernstein
E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in
the initiation Step of RNA interference. Nature 409: 363-366.
85. Nakanishi
K (2016) Anatomy of RISC: How do small RNAs and chaperones activate argonaute
proteins? Wiley Interdiscip Rev RNA 7: 637-660.
86. Ahlquist P (2002) RNA-dependent RNA
polymerases, viruses and RNA silencing. Science 296: 1270-1273.
87. Prat
AJ, MacRae IJ (2009) The RNA-induced silencing complex: A versatile
gene-silencing machine. J Biol Chem 284: 17897-17901.
88. Sui G,
Soohoo C, Affar el B, Gay F, Shi Y, et al. (2002) A DNA vector-based RNAi
technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U
S A 99: 5515-5520.
89. Knipe
DM Ed, Howley PM (2001) Field’s Viriology. 4th Edn. Lippincott Williams & Wilkins,
pp: 196-206.
90. Russell
SJ, Peng KW (2007) Viruses as anticancer drugs. Trends Pharmacol Sci 28:
326-333.
91. Wong
RSY (2011) Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin
Cancer Res 30: 87.
92. Dobbelstein
M (2004) Replicating adenoviruses in cancer therapy. Curr Top Microbiol Immunol
273: 291-334.
93. Ries
S, Korn WM (2002) ONYX-015: Mechanisms of action and clinical potential of a
replication-selective adenovirus. Br J Cancer 86: 5-11.
94. Robson
T, Hirst DG (2003) Transcriptional targeting in cancer gene therapy. J Biomed
Biotechnol 2003: 110-137.
95. Stetson
DB, Medzhitov R (2006) Type I interferons in host defense. Immunity 25:
373-381.
96. Devasthanam
AS (2014) Mechanisms under lying the inhibition of interferon signaling by
viruses. Virulence 5: 270-277.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Astronomy and Space Research
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)