920
Views & Citations10
Likes & Shares
Intellectual disability is the most common
neurodevelopmental defect in the world. This disorder affects 1-3% of the
general population. X-linked intellectual disability (XLID) is the frequent
form of intellectual disability which includes a heterogeneous group of
inherited disorders emerging as various degrees of intellectual disabilities.
Phenotypically, XLID is subdivided into syndromic (S-XLID) and non-syndromic
(NS-XLID) forms; where two-thirds of the XLID cases are thought to be
non-syndromic. Among the non-syndromic form, the aristaless-related homeobox
gene (ARX) is one of the ideal candidates to be evaluated in NS-XLID, since its
mutations are responsible for about 9.5% of XLID cases. Based on the previous
literature, mutations in the ARX gene influence the critical processes
associated with brain development. Our bioinformatics results showed that the
ARX is a highly conserved protein with a substantial role in an important
developmental pathway; and its deficiency can cause irreversible defects, mainly
in the brain, that leads to the development of XLID. Moreover, we addressed the
structural properties of the ARX protein to decipher the important role of the
ARX gene in the integrity of normal brain development.
Keywords: ARX, X-linked intellectual disability, Protein structure,
Wnt/β-catenin signaling
INTRODUCTION
Intellectual disability (ID) is the most
frequent neurodevelopmental disorder in the world characterized by an
intelligence quotient (IQ) below 70 [1]. The prevalence of ID is approximately
2-3% in the general population [2-8]. ID or associated phenotypes resulted from
a monogenic defect are subdivided into 4 categories according to the mode of
inheritance, autosomal dominant ID, autosomal recessive ID, X-linked ID and
mitochondrial ID [9-13]. Mutations in X-linked genes account for 5-10% of all
types of ID and are the most likely causes of ID in males [14].
ARX GENE: STRUCTURE
AND FUNCTION
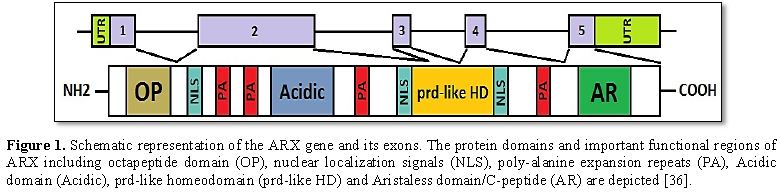
The Aristaless-related homeobox gene (ARX) is
located on the Xp22.13. It consists of 5 exons (Figure 1) and is transcribed into 2.8 kb mRNA. The structure of
the ARX protein consists of several different compartments, including [15-18]:
1) A highly conserved homeobox domain (repressor domain) that spans the amino
acids from 328 to 387. This domain directly binds to DNA [19]; 2) C-terminal
OAR or aristaless domain (activator domain) which spans the amino acids from
530 to 543 of protein (Figure 1)
[20]; 3) Octapeptid domain which is a receptor site beside the N-terminal of
the ARX protein for some enhancer proteins that contribute to ARX functional
activity adjustments [21]; 4) Four polyalanine tracts which are located between
Hundredth-degree amino acids and 115, 144 and155, 278 and281, also 432 and 440,
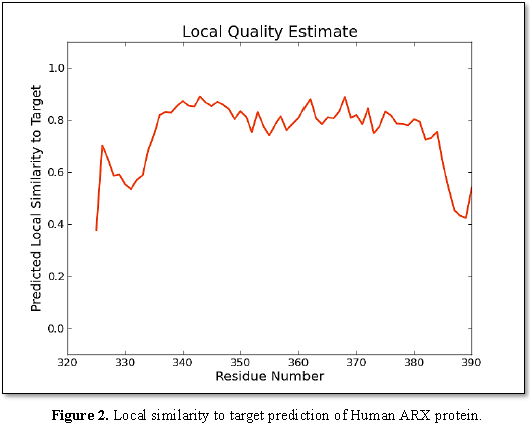
that each one has 16, 12, 7 and 9 residues, respectively [22-24]. It was determined that the ARX
gene is evolutionarily conserved in different species and according to Figure 2; it has a high local
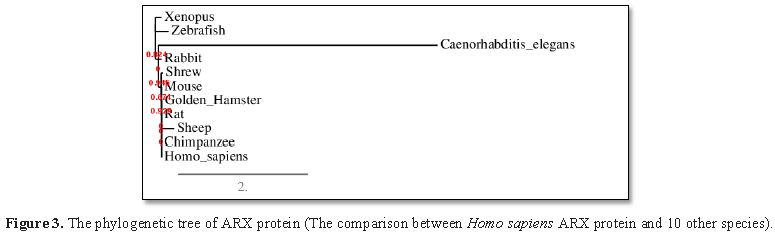
similarity to its target binding sites. Also, the sequence alignment of this
protein with other species (Figure 3)
confirms that the functional domains of ARX protein are highly conserved, thus
it has been predicted that the mutations of this gene can be highly pathogenic
[25-35].
ARX AND THE FREQUENCY OF ITS
MUTATIONS
According to European XLID consortium, mutations of
ARX gene has been found in 9.5% of families with X-linked intellectual
disability and 7.5% of large families with 2 or more affected males from
multi-generations that are related with each other through an obligate carrier
female [21,36-38].
ARX-ASSOCIATED
PHENOTYPES
ARX incapacitate mutations
through exons and introns and subsequently different domains, are associated
with a wide spectrum of phenotypes ranging from severe developmental
abnormalities of the brain to syndromic forms of XLID. Early infantile
epileptic encephalopathy-type1 (OMIM#308350); Lissencephaly-type2
(OMIM#300215); Hydranencephaly with abnormal genitalia (OMIM#300215); Proud
Syndrome (OMIM#300004); Partington Syndrome (OMIM# 309510); X-linked Mental
retardation and ARX-related (OMIM#300419) are the known various syndromic
phenotypes associated with ARX mutations [39-43]. Nonetheless, how different
mutations in this single transcription factor can produce different phenotypes
is not completely understood.
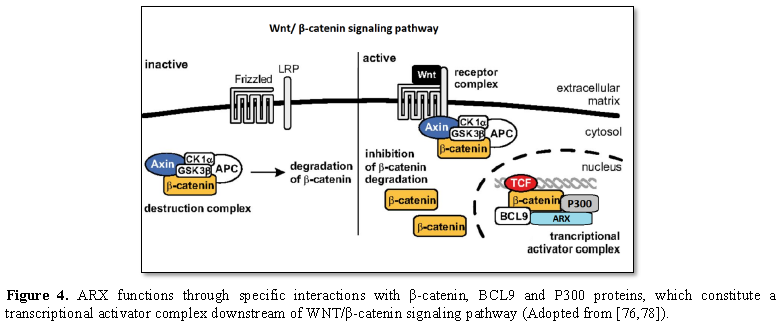
During the recent theory of
Il-Taeg Cho et al. [44], the ARX gene has interaction with different
cofactors/transcription factors and regulates single target genes in different
cell types. According to Il-Taeg Cho’s study, by using the proteomics method,
it was determined that the Wnt/β-catenin signaling pathway includes three
components such as B-cell CLL/lymphoma 9 (BCL9), β-catenin (CTNNB1) and
leucine-rich repeat flightless interacting protein 2 (LRRFIP2). They showed
that ARX positively controls Wnt/β-catenin signaling and that the C-terminal
domain of ARX interacts with the armadillo repeats of β-catenin to move forward
Wnt/β-catenin signaling.
Furthermore, they understood that P300 and
BCL9 also interact with ARX to adjust Wnt/β-catenin signaling. These data offer
new insights into how ARX can exclusively regulate cortical neurogenesis and
link the role of ARX with Wnt/β-catenin signaling [44] (Figure 4).
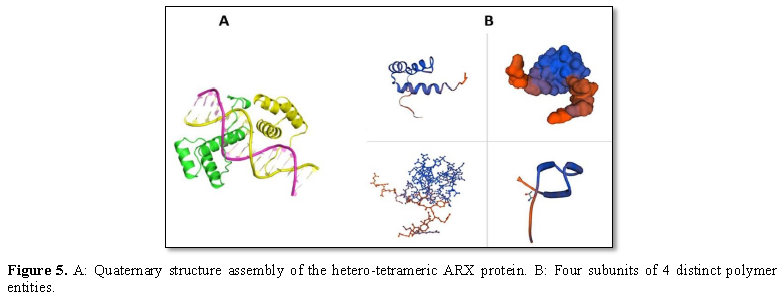
BIOINFORMATICS
ANALYSIS
To study the molecular features,
the structure of the ARX gene, bioinformatics analysis was performed using the
ExPASy tool and SWISS-MODEL server, respectively. The phylogeny tree of ARX
protein was also drawn using the software.
As illustrated in Figure 3, it was determined that the
ARX gene is evolutionarily conserved in different species. Moreover, the
sequence alignment of this protein with other spices confirms that the
functional domains of ARX protein are highly conserved, and therefore it has
been predicted that the mutations of this gene can be highly pathogenic.
WEB
RESOURCES
The
URLs for data offered here are as follows:
NCBI database (http://www.ncbi.nlm.nih.gov)
SWISS-MODEL
server (http://swissmodel.expasy.org)
Phylogeny software (http://phylogeny.lirmm.fr)
Expasy
software (http://www.expasy.org/)
Ensembl Genome Browser (http://www.ensembl.org)
1. Ropers
HH, Hamel BC (2005) X-linked mental retardation. Nat Rev Genet 6: 46-57.
2. Leonard
H, Wen X (2002) The epidemiology of mental retardation: Challenges and
opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 8:
117-134.
3. WHO
(2005) Atlas: child and adolescent mental health resources: Global concerns,
implications for the future. World Health Organization.
4. Durkin
M, Khan NZ, Davidson LL, Huq S, Munir S, et al. (2000) Prenatal and postnatal
risk factors for mental retardation among children in Bangladesh. Am J
Epidemiol 152: 1024-1033.
5. Durkin
MS, Hasan Z, Hasan K (1998) Prevalence and correlates of mental retardation
among children in Karachi, Pakistan. Am J Epidemiol 147: 281-288.
6. Durkin
M (2002) The epidemiology of developmental disabilities in low‐income
countries. Ment Retard Dev Disabil Res Rev 8: 206-211.
7. Costeff
H, Cohen BE, Weller L (1972) Parental consanguinity among Israeli mental
retardates. Acta Pediatr Scand 61: 452-458.
8. Bashi
J (1977) Effects of inbreeding on cognitive performance. Nature 266: 440.
9. Thompson
BL, Levitt P, Stanwood GD (2009) Prenatal exposure to drugs: effects on brain
development and implications for policy and education. Nat Rev Neurosci 10:
303-312.
10. Chaney
RH, Givens CA, Watkins GP, Eyman RK (1986) Birth injury as the cause of mental
retardation. Obstet Gynecol 67: 771-775.
11. King
BH, Dykens E. (1997) Mental retardation: A review of the past 10 years. Part
II. J Am Acad Child Adolesc Psychiatry 36: 1664-1671.
12. Piton
A, Redin C, Mandel JL (2013) XLID-causing mutations and associated genes
challenged in light of data from large-scale human exome sequencing. Am J Hum
Genet 93: 368-383.
13. Raymond
FL, Tarpey P (2006) The genetics of mental retardation. Hum Mol Genet 15:
110-R116.
14. Bassani
S, Zapata J, Gerosa L, Moretto E, Murru L, et al. (2013) The neurobiology of
X-linked intellectual disability. Neuroscientist 19: 541-552.
15. Bienvenu
T, Poirier K, Friocourt G, Bahi N, Beaumont D, et al. (2002) ARX, a novel
Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in
X-linked mental retardation. Hum Mol Genet 11: 981-991.
16. Chiurazzi
P, Tabolacci E, Neri G (2004) X-linked mental retardation (XLMR): From clinical
conditions to cloned genes. Crit Rev Clin Lab Sci 41: 117-158.
17. Ohira
R, Zhang HY, Guo W, Dipple K, Shih SL, et al. (2002) Human ARX gene: Genomic
characterization and expression. Mol Genet Metab 77: 179-188.
18. Poirier
K, Van Esch H, Friocourt G, Saillour Y, Bahi N, et al. (2004) Neuroanatomical
distribution of ARX in brain and its localisation in GABAergic neurons. Brain
Res Mol Brain Res 122: 35-46.
19. Kato
M, Dobyns WB (2004) X-linked lissencephaly with abnormal genitalia as a
tangential migration disorder causing intractable epilepsy: Proposal for a new
term, “interneuronopathy”. J Child Neurol 19: 392-397.
20. Friocourt
G, Poirier K, Rakic S, Parnavelas JG, Chelly J (2006) The role of ARX in
cortical development. Eur J Neurosci 23: 869-876.
21. Wigle
J, Eisenstat D (2008) Homeobox genes in vertebrate forebrain development and
disease. Clin Genet 73: 212-226.
22. Strømme
P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, et al. (2002) Mutations in the
human ortholog of Arista-less cause X-linked mental retardation and epilepsy.
Nat Genet 30: 441-445.
23. Kitamura
K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, et al. (2002) Mutation of
ARX causes abnormal development of forebrain and testes in mice and X-linked
lissencephaly with abnormal genitalia in humans. Nat Genet 32: 359-369.
24. Kato
M, Das S, Petras K, Kitamura K, Morohashi K, et al. (2004) Mutations of ARX are
associated with striking pleiotropy and consistent genotype-phenotype
correlation. Hum Mutat 23: 147-159.
25. Turner
G, Partington M, Kerr B, Mangelsdorf M, Gecz J (2002) Variable expression of
mental retardation, autism, seizures and dystonic hand movements in two
families with an identical ARX gene mutation. Am J Med Genet 112: 405-411.
26. Frints
SGM, Froyen G, Marynen P, Willekens D, Legius E, Fryns JP (2002) Re‐evaluation
of MRX36 family after discovery of an ARX gene mutation reveals mild
neurological features of Partington syndrome. Am J Med Genet 112: 427-428.
27. Grønskov
K, Hjalgrim H, Nielsen IM, Brøndum-Nielsen K (2004) Screening of the ARX gene
in 682 retarded males. Eur J Hum Genet 12: 701-705.
28. Reish
O, Fullston T, Regev M, Heyman E, Gecz J. (2009) A novel de novo 27 bp
duplication of the ARX gene, resulting from post-zygotic mosaicism and leading
to three severely affected males in two generations. Am J Med Genet A 149:
1655-1660.
29. Shinozaki
Y, Osawa M, Sakuma H, Komaki H, Nakagawa E, et al. (2009) Expansion of the
first polyalanine tract of the ARX gene in a boy presenting with generalized
dystonia in the absence of infantile spasms. Brain Dev 31: 469-472.
30. Nasrallah
MP, Cho G, Simonet JC, Putt ME, Kitamura K, et al. (2011) Differential effects
of a polyalanine tract expansion in Arx on neural development and gene
expression. Hum Mol Genet 21: 1090-1098.
31. McKenzie
O, Ponte I, Mangelsdorf M, Finnis M, Colasante G, et al. (2007) Arista-less related
homeobox gene, the gene responsible for West syndrome and related disorders, is
a Groucho/transducin-like enhancer of split dependent transcriptional
repressor. Neuroscience 146: 236-247.
32. Cho
G, Nasrallah MLP, Lim Y, Golden JA (2012) Distinct DNA binding and
transcriptional repression characteristics related to different ARX mutations.
Neurogenetics 13: 23-29.
33. Lin
W, Ye W, Cai L, Meng X, Ke G, et al. (2009) The roles of multiple importins for
nuclear import of murine arista-less related homeobox protein. J Biol Chem 284:
20428-20439.
34. Shoubridge
C, Tan MH, Fullston T, Cloosterman D, Coman D, et al. (2010) Mutations in the
nuclear localization sequence of the Arista-less related homeobox;
sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution
of the transcription factor and retards cell division. Pathogenetics 3: 1.
35. Dereeper
A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny. fr: robust
phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465-W469.
36. Takahashi
T, Fukuyama Y. (2008) Biology of seizures susceptibility in development brain.
2008: John Libbey Eurotext.
37. Cossée
M, Faivre L, Philippe C, Hichri H, Martin AdS, et al. (2011) ARX polyalanine
expansions are highly implicated in familial cases of mental retardation with
infantile epilepsy and/or hand dystonia. Am J Med Genet A 155: 98-105.
38. De
Brouwer AP, Yntema HG, Kleefstra T, Lugtenberg D, Oudakker AR, et al. (2007)
Mutation frequencies of X‐linked mental retardation genes in families from the
EuroMRX consortium. Hum Mutat 28: 207-208.
39. Poirier
K, Lacombe D, Gilbert-Dussardier B, Raynaud M, Desportes V, et al. (2006)
Screening of ARX in mental retardation families: Consequences for the strategy
of molecular diagnosis. Neurogenetics 7: 39-46.
40. Shoubridge
C, Fullston T, Gecz J (2010) ARX spectrum disorders: Making inroads into the
molecular pathology. Hum Mutat 31: 889-900.
41. Mandel
JL, Chelly J. (2004) Monogenic X-linked mental retardation: Is it as frequent
as currently estimated? The paradox of the ARX (Arista-less X) mutations. Eur J
Hum Genet 12: 689-693.
42. Bienvenu
T, Poirier K, Friocourt G, Bahi N, Beaumont D, et al. (2002) ARX, a novel
Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in
X-linked mental retardation. Hum Mol Genet 11: 981-991.
43. Abedini
SS, Kahrizi K, Behjati F, Banihashemi S, Ghasemi Firoozabadi S, et al. (2012)
Mutational screening of ARX gene in Iranian families with X-linked intellectual
disability. Arch Iran Med 15: 361-365.
44. Cho
IT, Lim Y, Golden JA, Cho G. (2017) Arista-less related homeobox (ARX) interacts
with beta-catenin, BCL9 and P300 to regulate canonical Wnt signaling. PLoS One
12: e0170282.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Astronomy and Space Research
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)