662
Views & Citations10
Likes & Shares
Men Breast Cancer (MBC) is uncommon and occurs after the age of 60 years.
Among men the prognosis is poor as the cause of a medical condition is
discovered at a late stage where infiltrating ductal carcinoma accounts for
70-90% of male breast cancers. The In situ but not invasive carcinoma is
exclusively ductal, and it accounts only 7% to 8% of the breast cancer cases in
males. It is observed that 50-75% of breast cancer cases are spread to lymph
nodes. The etiology of male breast cancer is unknown. An excess risk has been
associated with the testicular disorders, obesity, benign breast disease
including gynecomastia, Klinefelter syndrome, etc. Preliminary evidence
suggests that BRCA2 is a strong cause. The carriers of the males with BRCA2
mutation have an increased lifetime risk of breast cancer with 80-fold in it.
It is also known that there is also a risk of breast cancer associated with
undescended testes and it is also related to orchiectomy, orchitis, testicular
injury, etc., with an increasing number of children the decreasing trend in
risk was observed gradually. It is also known that Liver cirrhosis is
associated with increased levels of estrogens possibly via high levels of
endogenous estrogens.
Keywords: Alcohol, BRCA2,
Estrogens, Gynecomastia, Intraoperative, MBC, Mammography, Testicular,
Ultrasonography, Smoking
INTRODUCTION
The evidence known is that the analytical epidemiology of male breast
cancer is similar with the epidemiology of female breast cancer, with a
potential role of factors related to hormonal status. It is also known that the
relative hyper estrogeny in men is potentially linked to increased risk of
disease. It is also believed that Genetics may also play a role; the high risk
is linked to a familial history of breast cancer. There is also a major risk in
patients with Klinefelter's syndrome. It is known that these studies are not
only carried in Europe and America, they are also carried out in Africa. For
example a Stage, estrogen receptor status, treatment and survival of 29 men
with breast cancer attending the Breast Clinic was carried out in the
Johannesburg Hospital between 1976 and 1985 and were reviewed. Most patients
had loco-regionally advanced disease at presentation.
What are causes of male breast malignancy is a common question. Torre
et al. [4] and Jemal et al. [3] say that the risk factors associated with the
leading causes of cancer death include tobacco use in lung, colorectal, stomach
and liver cancer, overweight and obesity and physical inactivity in breast and
colorectal cancer and infection in liver, stomach and cervical cancer. So a
substantial portion of cancer cases and deaths can be prevented by applying
effective prevention measures, such as tobacco control. A meta-analysis of
alcohol drinking and cancer risk. Bagnardi et al. [6] suggest that the evidence
is provided by the epidemiological literature on the association between
alcohol consumption and the risk of 18 neoplasms. They performed a search of
the epidemiological literature from 1966 to 2000 using several bibliographic
databases. Meta-regression models were fitted considering linear and non-linear
effects of alcohol intake. They found that strong trends in alcohol consumption
risk were observed for cancers of the oral cavity and pharynx, esophagus and
larynx. Less strong direct relations were observed for cancers of the stomach,
colon and rectum, liver, breast and ovary. It was seen that these diseases,
showed significant increased risks in ethanol intake of 25 g per day. They also
demonstrated that there was no either significant or consistent relation for
cancers of the pancreas, lung, prostate or bladder. Boffetta and Hashibe [7]
put in more details that there is a causal association been established alcohol
consumption and cancers of the oral cavity, pharynx, larynx, esophagus, liver,
colon, rectum and in women breast; an association is suspected for cancers of
the pancreas and lung. There is evidence that suggests that the effect of
alcohol is modulated by polymorphisms in genes encoding enzymes for ethanol
metabolism. They give the examples like alcohol dehydrogenases, aldehyde dehydrogenases
and cytochrome (P450 2E1), folate metabolism and DNA repair. They conclude that
the mechanisms by which alcohol consumption exerts its carcinogenic effect have
not been fully defined, although possible events include; a genotoxic effect of
acetaldehyde, the main metabolite of ethanol, increased estrogen concentration,
which is important for breast carcinogenesis and also a role of solvent for
tobacco carcinogens. The other types of evidence include production of reactive
oxygen species and nitrogen species and changes in folate metabolism. Alcohol
consumption is increasing in many countries and is an important cause of cancer
worldwide. It is known that in the sub-Sahara regions men’s alcohol consumption
is well established but female consumption is also on the rise. More evidence
is known by other workers, for example, Castellsagué et al. [8] show that it is
not only alcohol consumption, but joint effects of tobacco smoking and alcohol
drinking, may lead to Cancer causing. They analyzed data from a series of 5
hospital‐based case‐control studies of squamous‐cell carcinoma of the esophagus
conducted in high‐risk areas in South America. A total of 830 case subjects and
1779 control subjects were included in the pooled analysis. All exposure characteristics
of amount, duration, cessation and type of alcohol and tobacco consumed were
strongly related to esophageal‐cancer risk in both sexes. Women had the same
exposure profile as men, but the magnitudes of the associations were lower than
were those among men. In their study it was evident that black‐tobacco smoking
was associated with a 2‐fold increased risk as compared with the smoking of
blond or mixed tobacco and more details showed that Alcohol and tobacco alone
were strongly related to the risk of esophageal cancer. Particularly the
history of simultaneous exposure to cigarette smoking and alcohol drinking had
a strong suggestive effect on risk for cancer. Worse still, it was the evidence
that a mixed exposure of heavy alcohol drinking and black‐tobacco smoking
identified the group with the highest risk for developing esophageal cancer
(odds ratio=107). They concluded that moderate cigarette smoking without
drinking and moderate alcohol drinking without smoking had a negligible effect
on esophageal-cancer risk. But, simultaneous exposure to the same moderate
amounts increased the risk 12 to 19‐fold in men and in women, respectively.
There was no specific evidence on the effect of smoking and alcohol on the
breast malignancy in men. In Denmark, France, Germany, Italy and Sweden, Guénel
et al. [9] investigated the role of alcohol drinking in male breast cancer
using data collected in a population-based case. In their study, the cases were
74 histologically verified male breast cancer patients aged 35-70 years. The
controls (n=1432) were selected from population registers and frequency-matched
to the cases by age group and geographic area. They checked for consistency, so
a separate analysis was conducted using as controls the patients with a rare
cancer other than male breast recruited simultaneously in the European study
(n=519 men). They found that the risk of developing breast cancer in men
increased by 16% (95% CI: 7-26%) per 10 g alcohol per day (p<0.001). An odds
ratio of 5.89 (95% CI: 2.21-15.69) was observed for alcohol intake greater than
90 g/day, as compared with light consumers (<15 g/day). They concluded that
the relative risk of breast cancer in men is comparable to that in women for
alcohol intakes below 60 g/day.
The estimated number of cancer cases and deaths attributable to alcohol
drinking in 2002 by the WHO sub region, based on relative risks of cancers of
the oral cavity, pharynx, esophagus, liver, colon, rectum, larynx and female
breast obtained from recent meta and pooled analyses and data on prevalence of
drinkers obtained from the WHO Global Burden of Disease project shows the fact
that there is a total of 389,100 cases of cancer. These are attributable to
alcohol drinking worldwide, representing 3.6% of all cancers (5.2% in men, 1.7%
in women). The corresponding figure for mortality is 232,900 deaths (3.5% of
all cancer deaths).
It is known that Men Breast Cancer (MBC) is an uncommon but it is a
serious problem in men. Carcinoma of the male breast accounts for 0.8% of all
breast cancers. A considerable debate exists concerning the prognosis of breast
cancer in male patients compared with that in female patients. Some studies
have observed worse prognosis for men; others suggested the higher mortality
rates were primarily due to delayed diagnosis. Borgen et al. [10] carried a
study of survival time from diagnosis with invasive disease to death resulting
from breast cancer of 58 men treated between 1973 and 1989 was compared with
survival of 174 women treated between 1976 and 1978 who were matched by stage
of disease and age at diagnosis. All patients were treated by mastectomy and
axillary dissection. The results showed the following: In tumors which were
less than 2 cm in 70% of cases and 55% were free of axillary metastases. The histology
of the tumors differed significantly by gender (p<0.05). Significantly more
men had estrogen receptor-positive tumors (87%) than did women (55%,
p<0.001). Survival at 10 years was similar for male and female patients.
Epidemiologic studies of breast cancer in men have provided insights into the
pathogenesis and etiology of breast cancer in both sexes. Thomas [11] points
out the facts that the incidence and mortality rates of breast cancer among
countries and racial and ethnic groups have been observed mainly in women but
these problems also occur in men. In women the fact clearly indicates that the
causes of these variations are primarily risk factors related to the female. It
is known that it occurs in women at the change in the rate of increase at the
usual age of menopause; the assumption is that the midlife change in the rate
of increase with age in women is due to the reduction in ovarian hormone
production at menopause. In men the risk of breast cancer goes on to occurring
as age increases. These data conflict with the conventional even in prognosis,
the wisdom shows that breast cancer in men carries a worse prognosis than the
disease in women. Individual carcinomas from both the male and female breast
are histologically indistinguishable, but histologic types of ductal origin
occur relatively more frequently in men than in women and those of lobular
origin are very uncommon in men, reflecting the absence of lobular structures
in the normal male breast. Spatz [12] clarifies more details in that breast
cancer occurs at an older age in men than in women, usually it presents as a
painless, central breast lump. It is known that male breast cancer is 100 times
less common than female breast cancer; however, the prognosis for men is worse
than that for women as has been said. This is because of the delay in
diagnosis. Men with breast cancer have a high prevalence of metastatic disease
at the time of diagnosis. So although Carcinoma of the male breast (MBC) it is
an uncommon phenomenon, it is accounting for less than 1% of all malignancies
in men. It represents a biologically heterogeneous disorder and its clinical
course may vary from indolent and slowly progressive to rapidly metastatic
disease. Most of the MBC current knowledge regarding its biology, natural
history and treatment strategies has been drawn from its female counterpart.
The information regarding the prognostic relevance of new molecular markers is
not fully understood.
The scientific study of infectious diseases and their causes of breast
cancer also have provided insights into the pathogenesis and etiology in both
sexes related to breast cancer. It is histologically not distinguishable that
individual carcinomas are types of ductal origin that are observed from both
the male and female breast, but these situations are more common in men than
women because it is clearly observed that the increase of age in men is a
causing the risk of breast cancer.
Between 1983 and 1987,the incident cases (n=227) were diagnosed and obtained from 10 population-based cancer
registries of the surveillance, epidemiology and end results program of the
National Cancer Institute. The Controls (n=300) were selected by random digit
dialing and from Medicare eligibility lists. The exposure status were defined
as ever having been employed in a job which was classified as involving
potential exposure to electromagnetic was assigned without knowledge of
case/control status. Demers et al. [13] found out the following: An elevated
risk was found for any job with exposure (odds ratio (OR)=1.8, 95% confidence
interval (CI) 1.0-3.7) and risk was highest among electricians, telephone
linemen and electric power workers (OR=6.0, 95% CI=1.7-21) and radio and
communications workers (OR=2.9, 95% CI=0.8-10). They found that the risk did
not vary with duration of exposed employment. The risk was highest among
subjects who were first employed in jobs with exposure before the age of 30
years and who were initially exposed at least 30 years prior to diagnosis.
These results were due to the theory that electromagnetic fields may be related
to breast cancer in men.
In the US Men account for less than 1% of all cases of breast cancer,
estimates for 1995 showed that there were only 1400 (0.76%) of the 183,400
cases of breast cancer in the United States that occurred in men. From the
above paragraph we have already discussed that prognosis in men is poor, but it
is easy to detect the breast cancer as men have so little breast tissue.
Infiltrating ductal carcinoma accounts for most cases (70-90%) of male breast
cancers. In situ but not invasive carcinoma is exclusively ductal and accounts
for 7% of cases. The spread to lymph nodes are observed in 50-75% of cases. In
Zambia we do not have the records.
History is known that the earliest reference to breast cancer that the
Edwin Smith Surgical Papyrus from Egypt contents, shows that MBC dates were
from 3000 to 2500 years B.C. Despite all this, knowledge relevant to many
aspects of the disease in men is still limited. Crichlow [14] adds on the fact
that carcinoma of the male breast is a rare neoplasm and comprises only 1% of
all breast carcinomas and less than 1.5% of malignant tumors in men. He adds on
that the important differences exist between the men and women in clinical
presentation and prognosis. Males present at a later age and often after a
longer delay. The tendency for ulceration of the overlying epidermis is far
greater in men than women. Prognosis appears to be worse overall for men.
Palade et al. [15] points out that in 20 years they registered 10 observations
of male breast cancer (MBC) represented 1.3% out of 767 patients with breast
cancer. Most men with breast cancer present with a mass in the breast, the
evaluation of which should include a tissue diagnosis. The adequate local 38
therapy includes total removal of the breast only if the presence of invasive
cancer is established.
The difficulty of discovering MBC is that it tends to occur at an older
age in men than in women as mentioned above, the problem is that it usually
presents itself as a painless, central breast lump. Although male breast cancer
is 100 times less common than female breast cancer, the 42 prognoses for men is
worse than that for women, probably because of delay in diagnosis. A small
share of breast cancer, those cases arising at a young age, causes due to the
inheritance of dominant susceptibility genes conferring disease with a high
risk. The survival rates for men and women are similar in the stage of
age-adjusted to 5 years, but comorbidities in older men lead to worse
prognosis. Mammography is the process of using low-energy X-rays to examine the
human breast for diagnosis and screening is required for palpable breast masses
in men. Block and Muradali [16] point out that mammography has a sensitivity of
92% and specificity of 90% for male breast cancer (n=104). Hsing et al. [17]
points out that the etiology of male breast cancer is unknown. Other writers
suggest that obesity increases the risk of male breast cancer, possibly through
hormonal mechanisms. The risk factors for male breast cancer include history of
the family, genes mutations age, radiations of the chest and altered
testosterone-estrogen levels (for e.g. due to liver cirrhosis, gonad
dysfunction, estrogen use, obesity). Preliminary evidence suggests that BRCA2
is a strong cause. However, it does not confer a substantially elevated risk of
ovarian cancer in contrast to BRCA1. Occurrence of male breast cancer, a rare
disease, peaks at age 71 years. Familial cases usually have BRCA2 rather than
BRCA1 mutations: That is what above is said. Occupational risks include high
temperature environments and exhaust fumes, but electromagnetic fields have not
been implicated as Demers et al. [13] have found hyper estrogenisation
resulting from Klinefelter's, gonadal dysfunction, obesity or excess alcohol,
all increase risk as does exposure to radiation, whereas gynaecomastia does
not. However, some workers think gynecomastia does lead to MBC. Thomas et al.
[18] suggest the following: an increased risk of breast cancer is most strongly
associated with undescended testes and is also related to orchiectomy,
orchitis, testicular injury, late puberty and infertility; a decreasing trend
in risk was observed with an increasing number of children. High blood
cholesterol, rapid weight gain, benign breast conditions and hesitancy obesity
are the relative risks that estimate the breast cancer in men. About 90% of
tumors are estrogen-receptor-positive, among them tamoxifen is a standard
adjuvant therapy, but some of the individuals could also benefit causes from
chemotherapy technique. In men, an increase in risk of breast cancer has been
associated with testicular pathology and dysfunction and a decrease in risk has
been related to high fertility, a history of prostate cancer, and exogenous
androgens. Whereas, an immunohistochemical analysis shows that the tumors are
positive only for progesterone and estrogen receptors more frequently in men
rather than women. It is also known that liver cirrhosis is associated with
increased levels of estrogens possibly via high levels of endogenous estrogens,
which increases the risk of breast cancer in men. Mostly men from United States
die from breast cancer rather than from testicular cancer; where 9355 men
diagnosed with breast cancer in the United States from 2004 to 2008, there were
1934 deaths, compared with 1758 deaths from the 39,641 cases of testicular
cancer. Rosenblatt et al. [19] think that the developing breast cancer were
greater in men with relative odds and who developed their mammary neoplasm
before the age of 45 with the first-degree relatives than in men with older
first degree affected relatives; the risk in men with an affected sister was
greater in those under age 60 than in older men. The problem of MBC may also
include Occupational risks which include: high temperature environments and
exhaust fumes, but electromagnetic fields have not been implicated. Hyper
estrogenisation resulting from Klinefelter’s, gonadal dysfunction, etc., also
increase risk as it exposure to radiation. However, two observations of
gynecomastia have been noted as a possible risk factor for MBC. Other workers
feel gynecomastia does not lead to MBC.
Workers like Weiss et al. [20] say the same but make it clearer. They
say that the suspected genetic factors in MBC include AR gene mutations, CYP17
polymorphism, Cowden syndrome and CHEK2. They add on by saying that the
epidemiologic risk factors for MBC include disorders relating to hormonal
imbalances, such as obesity, testicular disorders (e.g. cryptorchidism, mumps
orchitis and orchiectomy) and radiation exposure. They add on that the
suspected epidemiologic risk factors include prostate cancer, prostate cancer
treatment, gynecomastia, occupational exposures (e.g. electromagnetic fields,
polycyclic aromatic hydrocarbons and high temperatures), dietary factors (e.g.
meat intake and fruit and vegetable consumption) and alcohol intake. These
discussions bring out so many differences and also agreements and disagreements
concerning MBC in men.
In the European Institute of Oncology, Gennari et al. [21] performed a
study showing data in which p21Waf1 and p27Kip1 proteins were evaluated in a
series of male breast cancer patients. Their data also suggested that the
immunohistochemical evaluation of p21Waf1 and p27Kip1 expression in male breast
carcinomas may be a further useful marker for selecting patients who express
functional proteins that can be predictive for the most efficient endocrine
response. In searching for more conservative treatment, they introduced in their
clinical practice sentinel node biopsy, and if present, the sentinel node
biopsy of the internal mammary chain was noted. The potential clinical
implications of complete nodal staging were far-reaching and gave them a major
new opportunity to stratify male patients with breast cancer for appropriate
surgery as well as giving valuable prognostic information. They concluded that
MBC has biological differences compared with female breast cancer.
The familial aggregation of breast cancer in males also requires a
study. Rosenblatt et al. [19] investigated a population-based case-control
study. In their study the cases were ascertained from 10 surveillance,
epidemiology, and end results program registries in the United States between
1983 and 1986. Controls were identified by random-digit dialing and from lists
of Medicare recipients. In their study, the relative odds of developing breast
cancer were similar in men with affected paternal and maternal relatives and in
men with affected mothers and sisters. The risk chances of getting cancer in a
male increased with the number of affected relatives to the man. They confirmed
the fact that the development of breast cancer was greater in men with
first-degree relatives who developed their mammary neoplasm before the age of
45 than in men with older first-degree affected relatives; the enhancement of
risk in men with an affected sister was greater in those under age 60 than in
older men. Easton et al. [22] add on to this familial effect of MBC. They say
that Breast cancer is known to have an inherited component, consistent in some
families with autosomal dominant inheritance; in such families the disease
often occurs in association with ovarian cancer. Previous genetic linkage
studies have established that in some such family disease occurrence is linked
to markers on chromosome 17q. Their work reports the results of a collaborative
linkage study involving 214 breast cancer families, including 57 breast-ovarian
cancer families; this represents almost all the known families with 17q linkage
data. Their point is that under the genetic model used in the analysis, the
most estimate of the proportion of linked breast-ovarian cancer families was
1.0 (lower LOD-1 limit 0.79). In contrast, there was significant evidence of genetic
heterogeneity among the families without ovarian cancer, with an estimated 45%
being linked. These results suggest that a gene(s) on chromosome 17q accounts
for the majority of families in which both early-onset breast cancer and
ovarian cancer occur but that other genes predisposing to breast cancer exist.
By examining the fit of the linkage data to different penetrance functions, the
cumulative risk associated with the 17q gene was estimated.
How do we diagnose MBC? Diagnosis of the breast cancer in men is mainly
based on examination with clinical testing, followed by ultrasonography,
mammography, etc., whereas, the aspiration cytology makes it possible to
confirm the malignancy tumors. The intraoperative pathology examination
confirms malignancy with resection biopsy and makes wider excision possible
during the same procedure. The Presentation of the tumor is usually a lump or
nipple inversion, but is often late, with more than 40% of individuals having
stage III or IV disease. Most tumors are ductal type only but in situ only 10%
are ductal carcinomas. When survival is adjusted for age at diagnosis and stage
of disease, outcomes for male and female patients with breast cancer is
similar. Surgery is usually mastectomy with axillary clearance or sentinel node
biopsy. Because 90% of tumors are hormonal receptor positive, tamoxifen is the
standard adjuvant therapy. Indications for radiotherapy and chemotherapy are
similar to female breast cancer. For metastatic disease, hormonal therapy is
the main treatment, but chemotherapy can also provide palliation.
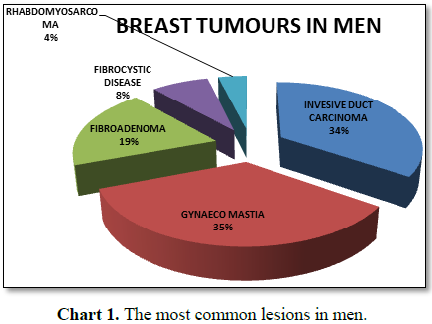
In ten years there were 9 invasive duct cell carcinoma male breast
patients. But there was one man, with a breast cancer which was a
Rhabdomyosarcoma. The youngest invasive malignancy was 17 years old and the
oldest man was 74 years old (Table 1 and
Chart 1). In our experience in the last six years from October 2011 to
November 2017 we had only seen this man as our man diagnosed with a breast
cancer [27,28].
Because of this case, as workers we felt it was essential to publicize
the following case.
CASE PRESENTATION
We present (F.K.) a case of a male patient who was 57 years old. He
complained of a breast lump on the right for 5 years that was gradually
increasing in size.
History of presenting
complaints
The lump was first noticed 5 years ago, and it was initially a small
lump on his breast. It was on the right and was not painful. It was said to be
gradually increasing in size but of little bother to the patient. Initially the
lump was regular, no associated nipple discharge and the skin above it was said
to be normal. But one year prior to presenting, the patient developed a whitish
foul-smelling discharge from right nipple. It was painful and later became more
irregular with shiny overlying skin.
The patient was married with five children; they were two boys and
three girls. His libido was said to be normal. The patient did not take alcohol
and gave no history of liver disease. He was HIV negative and his past medical
history was unremarkable.
REVIEW OF SYSTEMS
Cardiovascular system
Patient gave no history of chest pain, no shortness of breath, no pedal
swelling and he could lie flat without becoming breathless [29].
Respiratory system
Patient gave no history of cough, chest pain or coughing out blood
[30].
Gastrointestinal system
Patient gave no history of abdominal pain or distention, no yellowing
of eyes or body itchiness, no weight loss, constipation or blood in stool. The
Genitourinary and the Neurological systems were normal [31].
PAST MEDICAL HISTORY
He had no history of admissions for significant illnesses and he was
not on any treatment drugs like using anti-retroviral drugs, Cimetidine,
ketoconazole or testosterone antagonists.
Family history
There was a significant history of his mother who had a unilateral
gigantomastia. He was the eighth born out of 13 children which comprised of
three girls and ten boys.
Social history
The patient was a teacher by profession. He never smoked nor takes
alcohol.
GENERAL EXAMINATION
A middle-aged male was examined, his general condition was good. He was
of fair built and not in respiratory distress. He was not pale, jaundiced or
cyanosed and he was well hydrated. The left breast was normal (hypoplastic)
with no palpable lymph nodes in the left axilla. The examination of abdominal
case was normal: there was no caput medusae, no spider-naevi and liver span was
normal. Testicular examination revealed normal sized testicles. Prostate
examination reveals a normal sized prostate with normal texture. On the
examination of cardiovascular and respiratory organs, there was nothing
abnormal [32,33].
CLINICAL FINDINGS
Examination of the right breast: He had a breast tumor. The size was 4
× 5 cm, it was fixed to the nipple and overlying skin. There was nipple
retraction and nipple discharge. There was Peau d`orange appearance of the
overlying skin. The mass was hard, nodular and was moderately fixed to the
chest wall. Mobility was further reduced on tensing the pectoralis major
muscle. The weight was occupying all the four quadrants but was more prominent
in the upper outer quadrant. Axillary (right) examination revealed mobile lymph
nodes which were discrete. There were no palpable supraclavicular and cervical
lymph nodes (Figures 1 and 2).
OPERATIVE PROCEDURES
An Incision was made was made in normal skin with transverse elliptical
Incision. Dissection was done until the tumor was almost enucleated, what
remained was an area of fixation to the pectoralis major. The tumor was excised
together with a small part of the pectoralis major [34].
Level two lymph node dissection was done; lymph nodes were free and
mobile but were hard. The wound was washed with saline and a tube drain was
left in situ. The wound was closed in two layers with vicryl suture.
INVESTIGATIONS
Full blood count, cross match, liver function tests and renal function
tests were normal. The Chest X-ray was normal. Excisional biopsy and axillary
lymph node biopsy were done (post operatively) which revealed a ductal
carcinoma with lymph node metastases [35-37].
DISCUSSION
We accept that Breast cancer in men in Zambia is uncommon. In the
United States, their yearly diagnosed cases are 1500 new cases. We do not have
that data. We also know that Men Breast Cancer (MBC) often occurs at or after
the age of 60 years. Our patient presented himself at the age of 57. In men,
the prognosis is poor because it is discovered at a late stage, infiltrating
ductal carcinoma accounts for most cases (70-90%) of male breast cancers. In
our patient the tumor was part of the pectoralis major as it was being excised.
He had Level two lymph node at dissection; the lymph nodes were free and mobile
but were hard. Hsing et al. [17] point out that the etiology of male breast
cancer is unknown, although an excess risk has been associated with Klinefelter
syndrome, testicular disorders, benign breast disease including gynecomastia,
use of exogenous estrogens, radiation. Other writers suggest that obesity
increases the risk of male breast cancer, possibly through hormonal mechanisms.
They go on to say that the risk factors for male breast cancer include family
history, gene mutations age, chest radiation and altered testosterone-estrogen
levels (e.g. due to liver cirrhosis, gonad dysfunction, estrogen use, obesity).
Preliminary evidence suggests that BRCA2 is a strong cause [38].
In our patient there was a significant history of his mother who had a
unilateral gigantomastia, although he had no history of gigantomastia or
gynecomastia in his life its relationship may be or not be related.
CONCLUSION
The papers about MBC go on to say that, an increase risk in men of
breast cancer has been associated with testicular pathology and dysfunction and
Liver cirrhosis is also associated with increased levels of estrogens via high
levels of endogenous estrogens, increases the risk of breast cancer in men
[39].
1. Giordano
SH, Buzdar AU, Hortobagyi GN (2002) Breast cancer in men. Ann Intern Med 137:
678-687.
2. Parkin
DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2: 533-543.
3. Jemal
A, Bray F, Forman D, O'Brien M, Ferlay J, et al. (2012) Cancer burden in Africa
and opportunities for prevention. Cancer 118: 4372-4384.
4. Torre
LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, et al. (2015) Global cancer
statistics, 2012. A CA Cancer J Clin 65: 87-108.
5. Sasco
AJ, Lowenfels AB, Pasker‐De Jong P (1993) Review article: Epidemiology of male
breast cancer. A meta‐analysis of published case‐control studies and discussion
of selected etiological factors. Int J Cancer 20: 538-549.
6. Bagnardi
V, Blangiardo M, La Vecchia C, Corrao G (2001) A meta-analysis of alcohol
drinking and cancer risk. Br J Cancer 85: 1700-1705.
7. Boffetta
P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7: 2149-2156.
8. Castellsagué
X, Muñoz N, De Stefani E, Victora CG, Castelletto R, et al. (1999) Independent
and joint effects of tobacco smoking and alcohol drinking on the risk of
esophageal cancer in men and women. Int J Cancer 82: 657-664.
9. Guénel
P, Cyr D, Sabroe S, Lynge E, Merletti F, et al. (2004) Alcohol drinking may
increase risk of breast cancer in men: A European population-based case-control
study. Cancer Causes Control 15: 571-580.
10. Borgen
PI, Senie RT, McKinnon WMP, Rosen WP (1997) Carcinoma of the male breast:
Analysis of prognosis compared with matched female patients. Ann Surg Oncol 4:
385-388.
11. Thomas
DB (1993) Breast cancer in men. Epidemiol Rev 15: 220-231.
12. Spatz
MW (1988) Breast cancer in men. Am Fam Physician 38: 187-189.
13. Demers
PA, Thomas DB, Rosenblatt KA, Jimenez LM, McTiernan A, et al. (1991)
Occupational exposure to electromagnetic fields and breast cancer in men. Am J
Epidemiol 134: 340-347
14. Crichlow
RW (1974) Breast cancer in men. Semin Oncol 1: 145-152.
15. Palade
R, Vasile D, Grigoriu M, Roman H (1997) Breast cancer in men. Chirurgia 92:
159-165.
16. Block
WD, Muradali D (2013) Breast cancer in men. Can Med Assoc J 185: 1247.
17. Hsing
AW, McLaughlin JK, Cocco P, Chien HT, Fraumeni JF (1998) Risk factors for male
breast cancer (United States). Cancer Cause Control 9: 269-275.
18. Thomas
DB, Margarita Jimenez L, McTieman A, Rosenblatt K, Stalsberg H, et al. (1992)
Breast cancer in men: Risk factors with hormonal implications. Am J Epidemiol
135: 734-748.
19. Rosenblatt
KA, Thomas DB, McTiernan A, Austin MA, Stalsberg H, et al.(1991) Breast cancer
in men: Aspects of familial aggregation. JNCI 83: 849-854.
20. Weiss
JR, Moysich KB, Swede H (2005) Epidemiology of male breast cancer epidemiology.
Biomakers Prev.
21. Gennari
R, Curigliano G, Barbara A, Jereczek-Fossa, Zurrida S, et al. (2004) Male
breast cancer: A special therapeutic problem. Anything new? Int J Oncol 24:
663-670.
22. Easton
DF, Bishop DT, Ford D, Crockford GP (1993) Genetic linkage analysis in familial
breast and ovarian cancer: Results from 214 families. The Breast Cancer Linkage
Consortium. Am J Hum Genet 52: 678-701.
23. Mugala
DD, Benaya C, Simutowe M (2018) A breast cancer in a man in Ndola-Zambia: A
short case presentation. J Clin Case Rep 8: 1113.
24. Donegan
WL, Redlich PN (1996) Breast cancer in men. Surg Clin 76: 343-363.
25. Buzdar
AU (2003) Breast cancer in men. Oncology 17: 1361-1364.
26. Wooster
R, Neuhausen SL, Mangion J, Quirk Y, Ford D, et al. (1994) Localization of a
breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 265:
2088-2090.
27. Beyrouti
MI, Beyrouti R, Affes N, Frikha F, Abid M, et al. (2007) Breast cancer in men.
Presse Medicale 36: 1919-1924.
28. Rosenblatt
KA, Thomas DB, McTiernan A, Austin MA, Stalsberg H, et al. (1991) Breast cancer
in men: Aspects of familial aggregation. J Natl Cancer Inst I83: 849-854.
29. Fentiman
IS, Fourquet A, Hortobagyi GN (2006) Male breast cancer. Lancet 367: 595-604.
30. Sorensen
HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, et al. (1998) Risk of
breast cancer in men with liver cirrhosis. Am J Gastroenterol 93: 231-233.
31. Thomas
DB (1993) Breast cancer in men. Epidemiol Rev 15: 220-231.
32. Spatz
MW (1998) Breast cancer in men. Am Fam Physician 38: 187-189.
33. Thomas
DB, Jimenez LM, McTieman A, Rosenblatt KA, Stalsberg H, et al. (1992) Breast
cancer in men: Risk factors with hormonal implications. Am J Epidemiol 135:
734-748.
34. Fentiman
IS, Fourquet A, NHortobagyi G (2006) Male breast cancer. Lancet 367: 595-604.
35. Kelsey
JL (1979) A review of the epidemiology of human breast cancer. Epidemiologic
Rev 1: 74-109.
36. Friedman
LS, Gayther SA, Kurosaki T, Gordon D, Noble B, et al. (1997) Mutation analysis
of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet 60:
313-319.
37. Slamon
DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (1987) Human breast cancer:
Correlation of relapse and survival with amplification of the HER-2/neu
oncogene. Science 235: 177-182.
38. Iorio
MV, Ferracin M, Liu CG, Veronese A, Spizzo R, et al. (2005) microRNA gene
expression deregulation in human breast cancer. Cancer Res.
39. Boffetta
P, Hashibe M, La Vecchia C, Zatonski W, Rehm J (2006) The burden of cancer
attributable to alcohol drinking. Int J Cancer 119: 884-887.
40. Bezwoda
WR, Hesdorffer C, Dansey R, De Moor NMM, Derman DP, et al. (1987) Breast cancer
in men. Clinical features, hormone receptor status and response to therapy.
Cancer 60: 1337-1340.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Astronomy and Space Research
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)