2378
Views & Citations1378
Likes & Shares
Two hundred billion cells are dying every day. They need to be renewed. Dying or dead cell are involved a series of apoptotic events or eventually displays necrosis. Four basic modes of cell death have been identified. Apoptosis purges no-longer useful cells via phagocytosis, eliminating old cells, and/or stimulate tissue regeneration. Apoptotic cells are reflecting a suicidal event, whereas necrosis is playing role as a homicide. Active programed processes are characterized as being an autonomous happening. The process concerns caspases, which are inhibitors of apoptosis. According to morphologic and biochemical criteria, caspases contribute to membrane blebbing, intra-cytoplasmic alterations and formation of cysteine-rich domains. They are implicated in the translocation of nuclear proteins, play role in the control of the calcium efflux, regulate membrane permeability, and inhibit the translocation of mitochondrial membranes associated to apoptosis. DNA damages, nuclear fragmentation leading to the formation of apoptotic bodies, autophagic cell death, necrosis and other forms of cellular death are instrumental in the reliability of apoptotic events leading to cells death. Apoptosis appears to be crucial in morphogenesis, and actually guide a series of measurable biochemical features involved in tissue renewal and regeneration.
INTRODUCTION
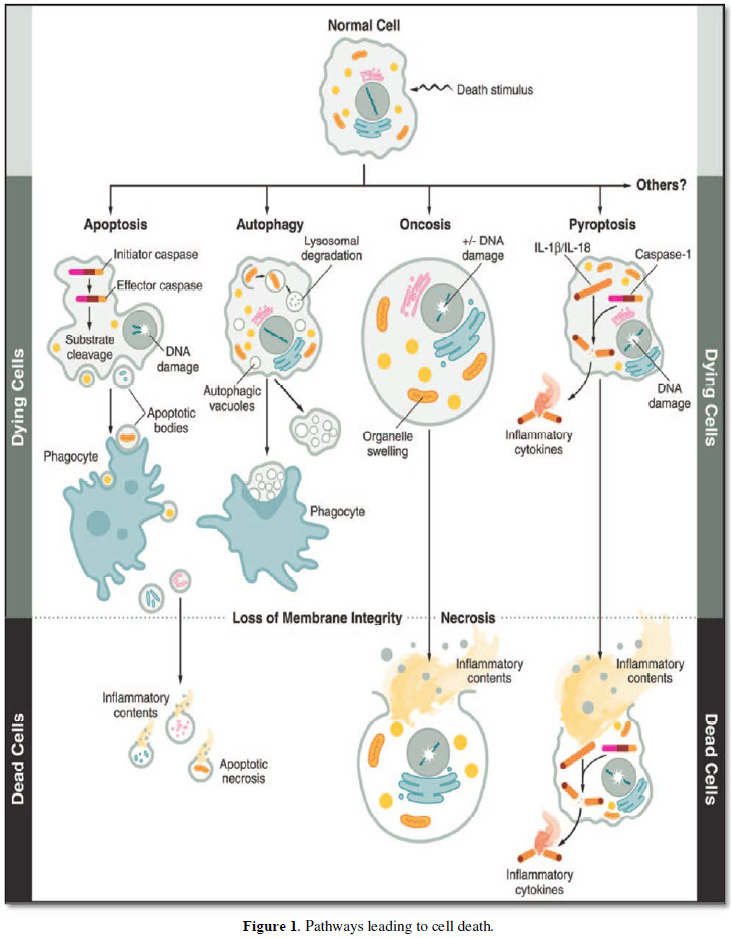
In 2009, the Nomenclature Committee on cell death proposed a set of recommendations for the definition of distinct cell death morphologies including ‘apoptosis’, necrosis’ and mitotic catastrophe [1]. A functional classification of cell death subroutes includes extrinsic apoptosis, caspase-dependent – or independent- intrinsic apoptosis, regulated necrosis, autophagic cell death and mitotic catastrophe. Functional classification of cell death modalities have established links with anoikis, autophagic cell death, caspase–dependent intrinsic apoptosis, caspase-independent intrinsic apoptosis, cornification, entosis, extrinsic apoptosis by death receptors, mitotic catastrophe, necroptosis, netosis, and pyroptosis [1] (Figures 1-3).
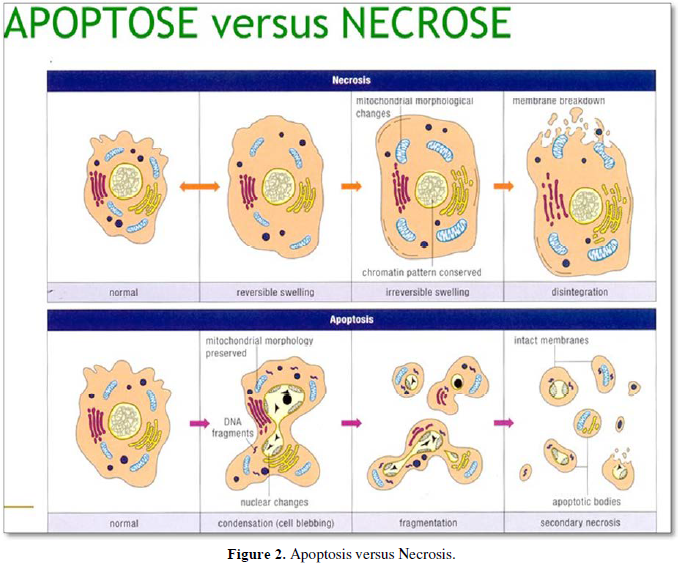
Apoptosis and necrosis comprise different modes of cell death, respectively implicated in cell withdrawal and recovery. Different types of cell death have been described according to morphologic and biochemical criteria [2]. Four basic modes of cell death have been identified following the biomedical literature, together with ad-hoc variants adapted to different situations [3].
Apoptosis purge no-longer useful cells from a tissue via phagocytosis, using scavengers and including macrophages. A crude estimation suggests that in the human body 60 billion cells die every day. The human body needs to yield 60-86.4 billion new cells per day, compensating the cell loss. Cell renewal implicates that ~ 200 billion of cells/per day die and therefore have to be renewed. Signals from apoptotic cells to scavengers indicates that apoptosis is a suicidal event, whereas necrosis is an homicide.
Control of proliferation or cell cycle implicates caspases, Bcl-2, inhibitor of apoptosis (IAPs), FAK, membrane blebbing (ROCK1), gelsoline, lamines A, B, C, β-caténine, ℽ-caténine, topoisomerase I and II. Extrinsic pathway of apoptosis involves death ligands (Fas L, TNF, TWEAK, TRAIL), activation of death receptors (intracytoplasmic death domain and cysteine-rich domain) and intrinsic apoptosis (DNA damage, ROS, stress response), Anti-apoptotic (BCL-2) anti- and pro-apoptotic events, pro-apoptotic (Bax) seems to play role in apoptotic events. Anti-apoptotic action of the Bcl-2 family inhibits the translocation of some nuclear proteins in the nucleus, control the calcium efflux from the rough endothelium, whereas antagonists of ROS control the permeability or inhibit the translocation of mitochondrial membranes, which are also implicated in this phenomenon.
Apoptotic Fragmentation
Specific biochemical analyses (such as DNA ladders) should not be employed as an exclusive means to define apoptosis, because this type of cell death may occur without oligonucleosomal DNA fragmentation. The presence of caspases or the cleavage product of their substrates is not sufficient to define apoptosis. The measurement of DNA fragmentation and/or caspase activation helps to better define the type of cell death, through the ‘intrinsic’ or the ‘extrinsic’ pathway, with or without the contribution of mitochondria.
Autophagy and Autophagic Cell Death
Degraded in phagosomes by caspases or cysteine-dependent aspartate specific proteases. Interleukin-1b xov-1. Initiator caspases (caspase-2,8,-9 and -10) contain a small prodomain. Activated effector caspases selectively cleave a restricted number of target proteins. One of the morphological and biochemical associated with apoptosis, the DNA ladder produced by cleavage of genomic DNA between nucleosomes generates fragments with length corresponding approximately to 180 base pairs [7].
Phosphatidylserine (PS) exposure is another caspase-dependent process is actively localized on the inner leaflet of the plasma membrane. Cytoplasmic and nuclear condensation, chromatin cleavage, formation of apoptotic bodies, maintenance of an intact plasma membrane and exposure of surface molecules target cell corpses for phagocytosis. Caspases -2, -6, -7, -8, -9 and -10 mediate the process of apoptotic cell death.
Signalization implicates the release of a number of signal molecules, implicated in cellular damages, and loss of adhesion. They are also involved in the release of a number of signal molecules, including receptors such as Fas/TNF, and glucocorticoids.
Extrinsic pathway of apoptosis: Death receptors are members of the tumor necrosis factor (TNF) family that include TNF-receptor-1 (TNFR1), CD95, death receptor 3, TNF-related apoptosis-inducing ligand receptor-1, and TRAIL-R2.
Intrinsic pathway of apoptosis: Depends on factors released from mitochondria. Caspase deficient mouse phenotypes
Deregulation of caspases underlines human diseases including cancer and inflammatory disorders.
Necrosis
Designate non-apoptotic accidental cell death. In the absence of phagocytosis, apoptotic bodies may lose their integrity and process to secondary or apoptotic necrosis..
Mitochondrial alterations, lysosomal changes, nuclear changes, lipid degradation, increase in the cytosolic concentration of calcium (Ca2+) result in mitochondrial overload and activation of caspase proteases. A crucial role for the serine/threonine kinase RIP1 has been demonstrated. Necrotic cell death is identified by the absence of apoptotic or autophagic markers, especially when the cells undergo early plasma membrane permeabilization. (Figures 1, 2).
Caspases are a family of genes (endoproteases) maintaining homeostasis through regulating cell death and inflammation (6). These endoproteases hydrolyze peptide bounds in a reaction that depends on catalytic cysteine residues. Caspase-3, -6, -7, -8, and -9 contains a caspase-recruitment domain (CARD), that displays a death effector domain.
Cornification
Pyroptosis
OTHER TYPES OF CELL DEATHS
A variety of cell death has been identified including:
Atypical cell death modalities, Mitotic catastrophe: Which can be accompanied by morphological alterations including micronucleation. Mitotic catastrophe can lead either to apoptotic morphology or to necrosis.
Anoikis: This form is induced by the loss of attachment to the substrate or to other cells. Excitotoxicity Wallerian degeneration
Neurons or axon degenerates without affecting the main cell body of the nervous system. Neurons affected by Wallerian degeneration remain alive.
- Galluzzi1 L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, et al. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differentiation 19: 107-120.
- Kroemer G, Galluzzi1 L, Vandenabeele P, Abrams J, Alnemri ES, et al. (2009) Classification of cell death. Cell Death Differ 16: 3-11.
- Liu X, Yang W, Guan Z, Yu W, Fan B, et al. (2018) There are only four basic modes of cell death, although these are many ad-hoc variants adapted to different situations. Cell Biosci 8: 1-12.
- Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and Necrosis: mechanistic description of dead and dying eukariotic cells. Infection and Immunity 73: 1907-1916.
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239-257.
- Mcilwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harbor Perspectives Biol 5: 1-28.
- Abe Ji, Murrell C (2016) Pyroptosis as a régulated form of necrosis. PI+/Annexin V- : High caspase 1 : Low Caspase 9 activity in cells = pyroptosis ?. Circulation Res 118 : 1458-1460.
- Bretnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology 14: 9-32.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)