610
Views & Citations10
Likes & Shares
Background: To assess the role of volumetric modulated arc therapy (VMAT) in management of advanced Hepatocellular carcinoma (HCC) patients and compare survival outcome with Best Supportive Care (BSC) as well as the response rate and toxicity of VMAT.
Method: Fifty patients were enrolled in the study and divided into two groups; arm (A) which is radiotherapy included 25 patients received radiation therapy 50.4 Gy in 28 fractions and arm (B) which is Best Supportive Care where patients received palliative care in the form of pain management, nutritional and liver support. Median age of the whole group is 56.5 years, the majority are males who are HCV positive carriers >90%. More than 50% are child Pugh (B), the rest are (A). According to BCLC staging 48% of the patients are stage C. Patients in both arms are closely similar regarding baseline clinical and pathological parameters.
Results: Median progression free survival (PFS) in arm A was 6.9 months versus 5.9 months for arm B and this was statistically significant with P-value=0.01, but median Overall Survival (OS) was equal in both arms (10 months in both) with P=0.5.The overall response rate (ORR) is 44% (1 patient had Complete Response (CR) and 10 patients had Partial Response (PR). Tumor response and performance status (PS) are the 2 most important prognostic factors that shows statistical significant difference with overall survival in arm (A), where patients with CR or PR had longer OS survival (12 months) compared to those with Stationary Disease(SD) (10 months) or Progressive Disease (PD) (4 months) with P-value 0.001. Also patients with PS 1 had longer survival (10 months) compared to those with PS 2 (6 months) with P-value 0.01.The most common toxicity with radiation was radiation induced liver disease (RILD) (28%) and the most important factor associated With the occurrence of RILD was the planning target volume (PTV) (P=0.02).
Conclusion: Radiotherapy with VMAT provides PFS advantage over BSC and achieved a good response rate in patients with advanced Hepatocellular carcinoma and patients who had a good response lived longer than patients who had poor response.
Keywords: Hepatocellular carcinoma, Radiotherapy, RapidArc, VMAT, Best supportive care
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related death in the world [1]. According to the results of national population based registry program of Egypt 2008-2011 Hepatocellular carcinoma is the most common prevalent cancer in males accounting for 33%, preceding bladder (10.7%),lung (6%) and prostate (4.2%).Surgery, provides survival rates 70% at 5 years, is appropriate in a small fraction of patients because of advanced stage at diagnosis [2].
Patients also can be treated with Trans Arterial Chemo Embolization (TACE), Radio Frequency Ablation (RFA), Percutaneous Ethanol Injection (PEI) and Targeted Agents. All these agents are used in early stages HCC and restricted to specific locations in the liver, and requires high cost and presence of co morbidities [3]. Portal vein tumor thrombosis (PVTT) is another issue that renders HCC tumors not applicable for the above mentioned therapies and so treatment options are extremely limited to only Best Supportive Care including pain management and liver support. It was reported short survival duration less than 3 months in patients diagnosed PVTT. The local control of PVTT helps to preserve liver functions and enables the implementation of various therapeutic options [4].
Radiotherapy is an option for this type of patients but it was limited by low tolerance dose of liver and occurrence radiation induced liver disease (RILD). A clinical syndrome characterized by ascites, anicteric hepatomegaly, and impaired liver function, usually occurs 2 weeks to 4 months after completion of Radiotherapy. It is affected by total dose to the liver and volume of irradiated normal liver. RILD is treated by supportive measures. In severe cases of RILD, hepatic failure may occur. The low tolerance dose of the liver limits the application of higher radiation doses to the tumor [5].
New techniques in radiotherapy have allowed higher doses to target the tumor while limiting the dose to normal liver tissue. More conformal types of radiotherapy have been developed to deliver highly conformal treatment with minimal damage to surrounding normal liver, including Intensity Modulated Radiotherapy (IMRT), Image Guided Radiotherapy (IGRT) and Stereotactic Body Radiotherapy (SBRT)
The availability of IMRT and the evolution of VMAT was a breakthrough in treatment of HCC patients. VMAT was formally used in metastatic liver lesions but then its use is extended to primary HCC. The role of VMAT became more obvious in treatment of HCC based on many studies:
Verbakel et al. [6] and Wagner et al. [7] compared RapidArc with IMRT for different malignancies and concluded that the major advantages of Rapid Arc over IMRT were the lower MUs and the shorter treatment time, which reduces the intra-fractional movement. Park et al. [8] study, treated advanced HCC patients with PVTT, both V30 and dose to organs at risk were lower in Rapid Arc compared to IMRT. Wagner et al. [7] reported that RapidArc in treatment of advanced HCC patients not amenable to surgery or local therapies yielded overall survival and local control benefit which makes it appropriate technique for management of these patients.
AIM OF WORK
The aim of this study is to assess the role of radiotherapy using RapidArc or VMAT technique in management of advanced HCC patients who are inoperable and not candidates for local ablative therapies and compare it with Best Supportive Care management regarding survival outcomes and to assess toxicity and response rate in the group of patients receiving radiation.
PATIENTS AND METHODS
The study was carried out at Kasr El Aini Center of Clinical Oncology (NEMROCK) after acceptance of our scientific and ethical committees and a written consent from all patients before their recruitment in the study. Fifty Patients with radiologically or pathologically proven HCC were assigned to receive either external beam radiotherapy EBRT using Rapid Arc technique or to receive supportive palliative care including management of pain, nutrition and liver support. The radiotherapy dose is 50.4 Grey (Gy) given in conventional fractionation of 1.8 Gy/fraction in 28 day duration.
PRETREATMENT EVALUATION
Includes:
· Radiologically or pathologically proven HCC
· Tumor medically inoperable or technically unresectable (vascular invasion, more than 5 cm, 3 nodules more than 3 cm)
· Tumor not amenable to TACE (Portal vein thrombosis or presence of arterio-portal fistula)
· Tumor not amenable to RFA(Tumors larger than 5 cm; Unsafe location relative to visceral organs, bile ducts and vessels or Poor coagulopathy profile)
· Recurrent tumor after TACE, RFA, alcohol and microwave ablation
· Absence of extra hepatic Metastases
Once patients fulfilled the inclusion criteria, baseline investigations are done:
Full medical history and physical examination, laboratory workup including AFP and CT scan or MRI abdomen and pelvis, chest X ray. Bone scan was done only in case of elevated Alkaline phosphatase (ALP) or symptomatic.
Radiological and Surgical consultation is done for patients in arm A to confirm ineligibility of surgery or ablative therapies before deciding radiation treatment.
STUDY DESIGN
Fifty patients with pathologically or radiologically proven hepatocellular carcinoma presented to Kasr el al. Ainy center of clinical oncology (NEMROCK) during the period from May 2014 to April 2016 were included in this study. They were divided into 2 groups: Arm (A) received radiotherapy by the RapidArc technique 50.4 Gy/28 fractions and Arm (B) received only palliative care in the form of pain management, nutritional and liver support. The study compared between both groups in clinical outcome as for survival and assessed response rate and safety in the radiation arm.
Follow up and response assessment
Clinical evaluations were planned during treatment at 1, 3, 6 months after treatment completion. Visits included laboratory assessment (Complete blood picture (CBC)-Kidney functions-Liver functions). Abdominal CT imaging was done every 3 months during the period of follow up. Tumor response was assessed using modified response evaluation criteria in solid tumors (M-RECIST) criteria.
Local recurrences or progression was defined by new enhancement or progressive disease with CT during follow up.
Liver toxicity and GIT toxicity were scored according to National Cancer Institute common toxicity criteria for adverse events (CTCAE version.3).
STATISTICAL METHODS
The OS and PFS were computed by the Kaplan-Meier method and compared by the log-rank test and the Cox proportional hazards model. P values less than 0.05 was considered statistically significant. The multivariate Cox model was used to study variation in the OS and PFS according to major baseline characteristics (age, sex, stage, histology and treatment). Statistical analyses were conducted using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Fifty patients with pathologically or radiologically proven Hepatocellular carcinoma presented to kasr El Ainy center of clinical oncology (NEMROCK) during the period from May 2014 to April 2016 were included in this study. They were divided into 2 arms: Radiotherapy arm received radiotherapy by the RapidArc technique 50.4 Gy/28 fractions and supportive care arm received only palliative care in the form of pain management, nutritional and liver support. The study compared between both groups in clinical outcome as for survival and assessed response rate and safety in the radiation arm. Twenty five patients were included in each arm and their main Clinical and pathological characteristics were balanced within the two groups with no statistically significant difference.
Regarding the response rate (RR) to radiotherapy, we have only 1 patient who entered in CR (4%) but progressed after a period of 9 months. Ten patients (40%) went into partial response where eight of them progressed later on during follow up and the other two lost follow up and censored. Accordingly, ORR reached 44%.Ten patients (40%) had stationary disease after radiation where nine of them progressed and one lost follow up and censored. Four patients (16%) progressed after radiation.
Only 2 factors were found to cause statistical significant difference in response rate to treatment which is (Table 1):
1) Portal Vein Thrombosis (PVT): Patients without portal vein thrombosis at presentation have higher overall RR (CR+PR) compared to the group with PVT. It was 8 (32%) patients with CR or PR at initial response vs. 3 (12%) patients only with (CR+PR). P-value: 0.01 for the rest of the PVT free group, there was only 2 patients with SD and no patients progressed. In the other group, there were 8 patients with SD and 4 others progressed directly after radiotherapy.
2) Barcelona Clinic Liver Cancer (BCLC) staging: Regarding patients with stage (A), all of them gave response after receiving radiotherapy (1 patient with CR and 8 patients with PR).For stage (B), 2 patients gave response and 4 patients had SD. And for stage (C), 6 patients had SD and 4 patients progressed, but none of them gave response with a statistically significant with P-value=0.001
The rest of the factors as child score, PS, presence or absence of cirrhosis and previous treatment intervention did not cause any statistical significant difference.
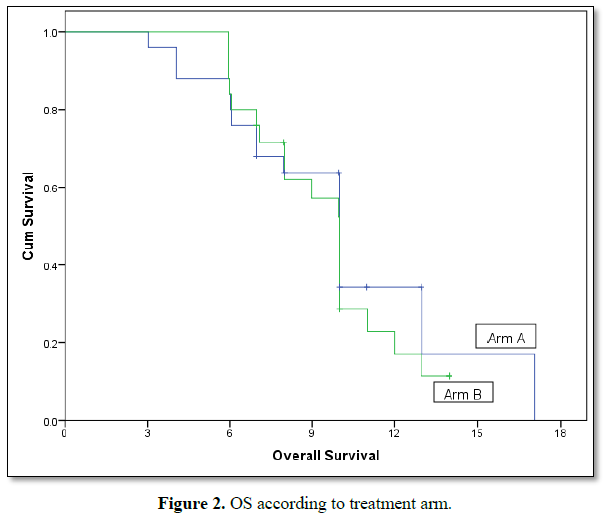
Median PFS in radiotherapy arm was 6.9 months versus 5.9 months for supportive care arm and this was statistically significant with P. value =0.01,but median OS was equal in both arms (10 months in both) with P. value=0.5 (Figures 1 and 2).
Several Clinical and pathological parameters are studied to see their correlation with survival of patients in both groups (Table 2).
Only two factors were found to cause statistically significant difference in the survival rate of group A:
1) Performance status: patients with PS (1) have median survival (MS) of 10 months vs. 6 months for those with PS 2 with p-value=0.015.
2) Response rate: median survival (MS) for the group of patients who gave response to radiation (CR or PR) was 12 months compared with 10 months for those with stationary disease (SD) and 4 months for patients who progressed (PD). P value=0.001.The rest of the factors didn’t impact OS significantly.
By multivariate analysis for the Performance status and response rate in arm (A) none of them was statistically significant (Table 3).
The previous factors were studied to see their effect on the other group who were managed with Best Supportive Care (BSC) (Table 4).
PS was the only variable strongly correlated to the survival of this group where we find that patients with PS (1) have MS of 10 months vs. 5.9 months for those with PS (2) which is statistically significant with P-value<0.01.The rest of the factors didn’t impact OS significantly.
Regarding safety, the most common toxicity was RILD representing 28% of the population that received radiotherapy. The factors studied associated with toxicity were mentioned in Tables 5, 6. We found that the “Mean volume of PTV was the only factor causing statistically significant difference in the occurrence of RILD. The mean volumes of PTV for the patients who developed RILD and those who didn’t were 620.2 and 579.7, respectively with P-value=0.028.
DISCUSSION
Several types of conformal radiotherapy have been developed to deliver high dose to the tumor with minimal damage to surrounding normal liver, including IMRT, IGRT and SBRT [9].
Our study, enrolled advanced HCC patients not amenable to surgery or any ablative therapies where the majority presenting with PVT. They were classified as being advanced or late stage or difficult to treat. We assessed the role of radiotherapy in management of this group of patients using the VMAT technique regarding clinical outcome as for survival in comparison to BSC as well as response rate and toxicity of VMAT.
In our study, overall survival and progression free survival for the group of patients who received radiotherapy was 10 months and 6.9 months respectively, while for the other group was 10 and 5.9 months respectively. Therefore OS in both arms is equal while PFS shows statistical significant difference bet the two arms although the difference was only one month (6.9 vs. 5.9), 95% CI (5.3-6.7) and P-value is 0.01.
Wang et al. [18] study on 138 patients with advanced HCC receiving radiation dose ranging from 44 to 66 Gy with conventional fractionation revealed MS of 10.3 months (95% CI: 7.2-13.3 months).
Overall survival at 1 year was 45% in Wang study which is much higher than our 1 year survival reaching 38% but on subgroup analysis of our study we found that for the patients with CR or PR, 1 year survival is more than 50%.
Survival Rate is positively influenced if combined modality treatment is given as shown by the following studies.
Krishnan reviewed studies of radiotherapy in to the liver after TACE, 1 year survival ranged from 42% to 94% for doses ranging from 30 to 66Gy [10]. Seong [11] demonstrated 158 patients treated with conventional fractionation scheme and in combination with TACE. One-year and 2 year OS was 40% and 20% with a median survival of 10 months, respectively.
Yoon [12] analyzed clinical outcome for 412 patients treated with TACE and 3D conformal radiotherapy for HCC with portal vein thrombosis. For these patients, median survival was 10.6 months with 42.5% survival rate at one year.
The above trials generally show higher 1 year survival rate compared to ours , maybe due to that combined modality gives better response rate compared to single modality and consequently better survival rate, on the other hand most of our patients received only radiotherapy and BSC upon progression and died shortly after progression [13]. The overall Response Rate in our study was 44% which is exactly the same as Min et al. [14] using IMRT and Jang et al. [15] using helical Tomotherapy where they achieved also RR 44%. In other studies, the response rate after radiotherapy with or without other local modalities was 40-76% in advanced HCC. The response rate to PVT has been approximately 46% after radiotherapy.
In our study the overall response rate is 44% as mentioned above representing CR (one patient 4%), PR (10 patients 40%), SD (10 patients 40%), PD (4 patient 16%).The dose of radiation has been shown to be important factor in several studies. A higher radiation dose (50 Gy or more) achieved a higher response rate. A higher total dose (>45-60 Gy) showed a higher survival rate [16].
Another important factor is when comparing the results from radiotherapy alone or radiotherapy combined with TACE, combined treatment achieved a better tumor response [17]. Some also showed that combined treatment was related to a better response with a difference of 20%, although the difference was not statistically significant. This was against our finding, where patients who received radiotherapy as only modality have a higher response rate (7 patients with PR and 1 patient with CR) than those received any prior treatment followed by radiotherapy (2 patients only with PR). Maybe it is attributed to the presence of PVT in many patients that received combined modality which negatively affects their response rate.
In the study of Wang et al. [18] (where there is a major difference between its sample size and ours (138 vs. 25 patients), percentage of response rate was as follow: CR in 12 patients (11%), PR in 58 patients (53%), SD 32 patients (29%), PD in 7 patients (6%), although 21 out of 138 patients were not assessed because they are dead or lost follow up.
Toxicity
Because of the advancement in radiation therapy techniques and proper dose constraints, GIT toxicity (stomach, duodenum) and spinal cord toxicity has been reduced, however RILD is still the most prominent complication in patients with hepatic radiation.
RILD is classified into two types as follows
Classic RILD where there is anicteric hepatomegaly, elevation of ALP level of at least two folds and nonmalignant ascites (between 2 weeks and 3 months after completion of radiotherapy) [19].
Non classic RILD where there is elevation of transaminases of at least fivefold the upper limit of normal or of the pretreatment level (grade 3 or 4 hepatic toxicity of Common Toxicity Criteria Version 2.0 by National Cancer Institute) in the absence of documented progressive disease [20].
Majority of our patients are HCV carriers and cirrhotic, thus hepatocytes are more susceptible to radiation injury. The most common important toxicity is RILD occurred in 7 patients (28%). After studying several factors associated with RILD, we found that as the mean PTV volume increases, the higher risk of occurrence of RILD, where the mean of PTV volumes for the 7 patients who developed RILD was 620 cm3 vs. 579.7 cm3 for the 18 patients who were RILD free, with P-value=0.028.
Min et al. [13] reported that hepatic toxicity increases as the irradiated dose to normal liver increase. In the study RILD occurred in 12 patients (44%) of the population and mean dose to normal liver 22.5 Gy. Cheng et al. [21] also reported that mean liver dose of patients with RILD was significantly higher than those without (25 Gy vs. 19.65 Gy, P-value 0.02).
Pan et al. [22] recommended that the mean normal liver dose should be less than 28 Gy in 2 Gy fractions for primary liver cancer [22].
Similar dose constraint to normal liver used in our study which was even lower than the previously mentioned. We used 24 Gy as maximum tolerance dose to the liver based on the Quantec model.
Compared to the above studies , it is clear that our mean dose to normal liver minus PTV was 15.8 Gy ± 5.1 was lower than the previously mentioned in the above studies and so it has no significant difference in the occurrence of RILD with P-value=0.14.
Combined modality treatment is another factor to be correlated with RILD, where we can find in many studies that radiotherapy combined with TACE or non-selective hepatic arterial chemotherapy gives a higher rate of hepatic toxicity than radiotherapy alone [23-25]. No statistical significant difference was observed in the occurrence of RILD between those who received combined modality and those who did not, maybe due to small sample size and even less number of patients who underwent previous treatments or it is related to multiplicity of local treatments received by the patients in these studies which higher the toxicity compared to our patients who received only single modality prior to radiation.
The value of V30 was found to play an important role in the development of RILD in patients treated with conventional radiotherapy [26]. Kim et al. [26] also reported that the low dose coverage V5 and V10 were associated with toxicity but the potential risk of RILD by low dose radiation is still unclear.
Also the value V20 was significant parameter for development of RILD after conventional radiotherapy as reported by Liang et al. [27].
In a recent study of Cheng et al. [28], where it compared between the 3 techniques CRT, IMRT, Rapid Arc in treatment of advanced HCC, found that RapidArc was superior at the risk of RILD in consideration of lower V20 and V30. On the other hand, similar comparative study it was reported that rapid arc has higher V10 and mean dose compared to IMRT which should be taken with caution when treating HCC patients since it is associated with RILD as mentioned before.
Regarding our study, no significant difference was shown in the occurrence of RILD with V10, 20 and 30, which is probably due to the lower mean dose to normal liver (15.8 Gy) and as a result the mean of V10, 20, 30 will also be lower compared to other studies.
In addition to dose-related factors affecting RILD, Cheng et al, reported that patients with Child Pugh-B or hepatitis B virus (HBV) are also at a significant risk of developing RILD. Patients with CP-B had worse hepatic insufficiency compared with those with CP-A [29,30]. CP-B has a higher hepatic toxicity compared with CP-A. This was not obvious in our study due to our smaller sample size which failed to show statistical significant difference between both groups. HBV rather than hepatitis HCV infection was also associated with higher RILD. Because HBV carriers have poor tolerance to partial liver irradiation [29-31]. The group of patients who received radiation in our study, none of them had isolated HBV infection, the majority were HCV carriers and 3 patients had Co-infection B and C and though we could not assess HBV as a separate entity and there was no statistical significance also.
Limitations of our study include small sample size; relatively coarse 5 mm slice thickness and lack of a specific strategy to compensate for liver motion due to respiration. Respiratory gating techniques are not available in the department. Also abdominal compression and breath controls are not easily feasible in our patients.
The potential displacement of liver could be as large as 2-2.5 cm [32], it is suggested to incorporate motion compensation into traditional definition of margins.
In conclusion, RapidArc obtained favorable response rate, also provides PFS survival advantage over Best Supportive Care for the category of advanced HCC tumors who are not candidate for surgery or loco regional therapies.
SUMMARY AND CONCLUSION
We conclude that Radiation therapy provides better local control and overall response rate for patients who received radiation. Tumor response and performance status were the 2 most important prognostic factors that showed statistical significant difference with overall survival in arm (A) receiving radiotherapy. Patients with CR or PR had longer OS survival (12 months) compared to those with SD (10 months) or PD (4 months). Also patients with PS 1 had longer survival (10 months) compared to those with PS 2 (6 months) with P-value 0.01.
All patients with stage (A) according to the BCLC staging system, achieved better response rate (CR or PR) compared to stage (B) or (C), also patients without PVT achieved better response than those presenting with PVT.
The most common toxicity with radiation is RILD (28%) and the most important significant factor associated With RILD is the PTV volume.
Radiotherapy with VMAT is superior to BSC in PFS and achieved a good response rate in patients with advanced Hepatocellular carcinoma and patients who had a good response lived longer than patients who had poor response.
1. Bosch FX, Ribes J, Diaz M, Cleries R, et al. (2004) Primary liver cancer: Worldwide incidence and trends. Gastroenterol 127: S5-S16.
2. El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the united states. N Engl J Med 340: 745-750.
3. Guy J, Kelley RK, Roberts J, et al. (2012) Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol 10: 354-362.
4. Yamada K, Izaki K, Sugimoto K et al. (2003) Prospective trial of combined transcatheter arterial chemoembolization and three dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 57: 113-119.
5. Tse RV, Hawkins M, Lockwood G, Kim JJ, Knox J, Sherman M, Dawson LA (2008) Phase I study individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 26: 657-664.
6. Verbakel WF, Cuijpers JP, et al. (2009) Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int. J. Radiat Oncol Biol Phys 74: 252-259.
7. Wagner D, Christiansen H, Wolff et al. (2009) Radiotherapy of malignant gliomas: Comparison of volumetric single arc technique (RapidArc), dynamic intensity-modulated technique and 3D conformal technique. Radiother Oncol 93: 593-596.
8. Park JM, Kim K, Chie EK, Choi CH, Ye SJ, et al. (2012) Rapid Arc vs. intensity-modulated radiation therapy for hepatocellular carcinoma: A comparative planning study. Br J Radiol 85: e323-29.
9. Wu MC, Wang Y, Sun YF, Chen KJ, et al. (2006) Surgical management of small intrahepatic lesions adjacent to the major vasculature Zhonghua Wai Ke Za Zhi 44: 1631-1633.
10. Krishnan S, Dawson LA, Seong J, et al. (2008) Radiotherapy for hepatocellular carcinoma: An overview. Ann Surg Oncol 15: 1015-1024.
11. Seong J, Park HC, Han KH, et al. (2003) Clinical results of 3 dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma in the cirrhotic patients. Hepatol Res 27: 30-35.
12. Yoon SM., Lim YS, Won HJ, et al. (2012) Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: Long-term patient outcomes. Int J Radiat Oncol Biol Phys 82: 2004-2011.
13. Kangl MK, Kim MS, et al. (2011) High dose radiotherapy with intensity modulated radiation therapy for advanced hepatocellular carcinoma. Jpn J Clin Onc Tumo Ri 97: 724-731.
14. Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, et al. (2005) Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 23: 8739-8747.
15. Kim DY, Park W, Lim DH, Lee JH, Yoo BC, et al. (2005) Three dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer 103: 2419-2426.
16. Seong J, Lee IJ, Shim SJ, Lim do H, Kim TH, Kim J, et al. (2009) A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 29: 147-152.
17. Seong J, Keum KC, Han KH, Lee DY, Lee JT, et al. (1999) Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 43: 393-397.
18. Wang R, Zhao N, Li S (2013) MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2 and CDC42. Hepatol 58: 642-653.
19. Lawrence TS, Ten Haken RK, Kessler ML, et al. (1992) The use of 3D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys 23: 781-788.
20. Trotti A, Byhardt R, Stetz J, et al. (2000) Common Toxicity Criteria: Version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47: 13-47.
21. Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, et al. (2002) Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys 54: 156-162.
22. Pan CC, Kavanagh BD, Dawson LA, et al. (2010) Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 76: S94-S100.
23. Dawson LA, Normolle D, Balter JM, et al. (2002) Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 53: 810-821.
24. Liang SX, Huang XB, Zhu XD, Zhang WD, Cai L, Huang HZ, et al. (2011) Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with Child-Pugh Grade A cirrhosis. Radiother Oncol 98: 265-269.
25. Shim SJ, Seong J, Lee IJ, Han KH, Chon CY, Ahn SH (2007) Radiation-induced hepatic toxicity after radiotherapy combined with chemotherapy for hepatocellular carcinoma. Hepatol Res 37: 906-913.
26. Kim TH, Kim DY, Park JW, Kim YI, Kim SH, et al. (2006) Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol 29: 568-575.
27. Rongrui L, Na H, Zongfang L, Fanpu J, Shiwen J (2009) Epigenetic mechanism involved in the HBV/HCV-related hepatocellular carcinoma tumorigenesis. Curr Pharma Design 20.
28. Cheng D, Wang R (2014) A comparison between liver protection among 3D conformal radiotherapy, IMRT and Rapidarc for hepatocellular carcinoma. Radiation Oncol 9: 48.
29. Cheng JC, Wu JK, Lee PC (2004) Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys 60.
30. Jung J, Yoon SM, Kim SY, et al. (2013) Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 8: 249-253.
31. Cheng JC, Liu HS, Wu JK, Chung HW, Jan GJ (2005) Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys 62: 1150.
32. Cheng JC, Liu HS, Wu JK, Chung HW, Jan GJ (2005) Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys 62: 1150.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- BioMed Research Journal (ISSN:2578-8892)