988
Views & Citations10
Likes & Shares
In this study, the objective response was obtained in 12 cases of hepatocellular carcinoma (HCC) following chemotherapy and traditional medicine. Among them, the clinical data for ten patients with HCC were previously described, and 2 cases were continuous to be present here. During the schedule of drug administration, 12 cases of HCCs were treated using the different protocol of chemotherapy in conjunction with traditional medicine. One HCC accompanied with colon polyps obtained complete remission via hepatectomy and targeting oncogenic receptor tyrosine kinase inhibitor sorafenib. And, an approach to hepatic artery intervention chemotherapy plus cantharidin was carried out in another advanced HCC. All 12 patients with disease-free survival were 2, 2, 8, 6, 1.6, 1.8, 10, 15, 20, 20, 5 years, respectively. In discussion, the incidence of HCC is on the rise due to the impact of HBV and HCV, an additional recent data implicate hepatocyte growth factor (HGF)and HGF receptor oncogenic signaling (also HGFR/met oncogenic receptor),which act as a trigger for liver regeneration even in (hepatocellular) carcinogenesis.
Keywords: HCC, HBV, HCV, HGF/met or met oncogenic receptor 5-fluorouracial, Traditional herbal medicine
INTRODUCTION
In clinical situation only 5.3% of patients who were belonged to the indication of hepatectomy whereas 90% of them were conclusively the protocol of chemotherapy. One of this approaches, traditional medicine occupied its important role in the field of hepatocellular carcinoma treatment. In search for the effective approach of PHC [1-6], we had summarized the retrospective study of HCC under remission, with the combined protocol of chemotherapy and traditional medicine.
METHODS AND RESULTS
The clinical data from case 1 to case 10 have been previously reported [7,8]. The detail results of ten patients described in Table 1.
CASE REPORTS
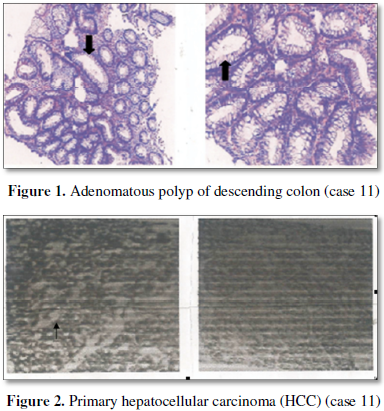
Case 11: A 63 year old man was diagnosed as his hepatic tumor due to his occasional colon polyp examination (Figure 1). At CT examination on August 23, 2012 and September 1, 2012 respectively showed 2.0 × 2.4 cm tumor in the right anterior lobe of his liver. Moreover, on B ultrasound on September 3, 2012 and December 13, 2012 respectively also consistently showed 26 × 17 mm mass in the right anterior lobe of his liver. Histologically, primary hepatocellular carcinoma (PHC) was further diagnosed after biopsy of liver tumor tissue on April 26, 2013 (Figure 2). The remainder of liver enzyme performed serum AFP 1.74 ng/ml (control 0-7.0 ng/ml). He had a past history of viral hepatitis B (HBV) infection. The patient was once undergoing radio-frequency ablation of HCC on September 4, 2012; and repeat CT examination the tumor size was increased to 33 × 31 mm on April 15, 2013 and 33 × 37 mm liver nodule on June 17, 2013, respectively. In May, 2014 he was therefore given his hepatectomy (at right lobe), with the combination treatment of Sorafenib tablets (initial dosage 2#/day × 5 months, then 1#/day intermittent until to 1.5 years) and antivirus Entecavir despensible tablets. In the follow up, he was remained well with over 5 year’s survivor.
Case 12: A 41 year old man stated that he was disgusted with taste of greasy, accompanied with nausea and vomiting for one month, and chief complaint of abdominal mass one day ago. At physical examination showed 6 × 5 cm harden mass in his hepatic lesion, and an enlarged liver was felt 7 cm below the right costal margin. Laboratory data included serum AFP 40.59 ng/ml (control 0-7 ng/ml), CEA 5.86 (control<5.0 mg/L), CA-125 63.0 (control<35.0 u/ml), CA-159 91.31 (control<35.0 ku/L), serum HBsAg (-), serum HBsAb 53.3 (+), serum HBeAg (-), serum HBeAb 1.29 (+), serum HBcAb 1.45 (+). He was diagnosed as advanced HCC. Treatment consisted of hepatic artery intervention chemotherapy (Lobaplatin plus camptothecin injection, Figure 3) in another provincial tumor hospital and demethylcantharidin tablets. He was in stable disease for 3 months and now being treated in other hospital.
DISCUSSION
Sorafenib is an oral inhibitor of oncogenic receptor tyrosine kinases, including VEGFR, PDGFR, KIT, FLT3 and also its activity against c-Raf and b-Raf [9-22]. There were several clinical trials of sorafenib evaluated in HCC in European centers [23], in Asian and Pacific centers [24] and in Hong Kong [25]. Subgroup analysis indicated a benefit for sorafenib in stable disease. Compared with those HCC with hepatitis B, those HCC with hepatitis C had longer time to progression [26-32]. Moreover, in those with HBV, the HCC without extrahepatic metastasis, particularly the absence of lung metastasis, predicted clinical benefit [33]. At present, a durable complete remission of an earlier HCC with the combination of hepatectomy and sorafenib in a third-line setting, in the follow up, that has offered a progression-free survival of 5 years.
Steroid androgen is another environmental revelance factor of breast tumors and hepatoma. Up to now, the development of at least 22 hepatic tumors had been well documented in patients receiving androgenic steroid (oxymetholone, methyl testosterone) therapy, and promotion of murine hepatocarcinogenesis by testostertone is androgen receptor- dependent [34,35]. Regression of the hepatic tumor has been reported in four cases after cessation of therapy. Bone marrow transplantation from a histocompatible sibling was advocated by successful hematopoietic and immunological reconstitution and associated with regression of the hepatic masses in a 13 year old boy with aplastic anemia. In clinical study, Zhu [36] previously reported that androgen methyl testosterone can induce breast tumors in one patient with severe aplastic anemia, which implicate that androgen via its oncogenic androgen receptor (AR) signaling had oncogenic potential.
More recent, HGF-HGF receptor (met oncogenic receptor) oncogenic signaling might play an important role in HCC, which implicate its target therapy. Foretinib, the first multi-target c-met TKI to under investigation, produced a promising benefit in HCC patients [37]. The chemically-modified monovalent antibody DN30 was found to inhibit ligand-independent activation of the met oncogenic receptor, providing another target therapy [38,39]. This is testable.
ACKNOWLEDGMENT
The authors are thankful and cherish the memory of Professor Yang BY for providing his previous data (case 1 to 4) and Dr. OU GD for providing his HCC data (case 11) in preparation of this manuscript.
The authors confirm that this article content has no conflict of interest.
1. Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: Worldwide incidence and trends. Gastroenterology 127: S5-S16.
2. Irabor DO, Alese OB (2009) Surgical management of spontaneous rupture of primary liver cell carcinoma in a tropical low socio-economic population: A case report. JCCM 4: 332-334.
3. Chisari FV, Filippi P, Buras J, Mclachlan A, Popper H, et al. (1987) Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci U S A 84: 6909-6913.
4. Giaglia JL, Antonia SJ, Berk LB, Bruno S, Dessureault S, et al. (2010) Systemic therapy for advanced hepatocellular carcinoma: Past, present and future. Cancer Control 17: 120-129.
5. Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV (1992) Hepatocyte growth factor inhibits growth of hepatocellular carcinoma. Proc Natl Acad Sci U S A 89: 373-377.
6. Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, et al. (1987) Molecular cloning and expression of human hepatocyte growth factor. Nature 1987: 342, 440.
7. Zhu G, Musumeci F, Byrne P, Gupta D, Gupta E (2017) Role of traditional herbal medicine in the treatment of advanced hepatocellular carcinoma (HCC): Past and future ongoing. Adv Pharm J 2: 115-120.
8. Zhu G, Musumeci F, Byrne P, Gupta D, Gupta E (2017) Treatment of advanced hepatocellular carcinoma (HCC) with the combined protocol of chemotherapy 5-Fluorouracil and traditional medicine: Report of ten cases. Clin Trials Pathol Case Stud 2: 1-5.
9. Link JS, Bateman JR, Paroly WS, Durkin WJ, Peters RL (1977) 5-Flourouracil in hepatocellular carcinoma: Report of twenty-one cases. Cancer 39: 1936-1939.
10. Porta C, Moroni M, Nastasi G (1995) 5-Fluorouracil and d,1-leucovorincalcium are active to treat unresectable hepatocellular carcinoma patients: Preliminary results of a phase II study. Oncology 52: 487-491.
11. Jiang W, Lu Z, He Y, Diasio RB (1997) Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: Implication in 5-fluorouracil-based chemotherapy. Clin Cancer Res 3: 395-399.
12. Zhu G (1992) Oncogenic receptor hypothesis (1989-1991). Voice of America (VOA) 12: 31.
13. Zhu G, Musumeci F, Byrne P (2013) Induction of thyroid neoplasm following plant medicine marine algae (sargassum): A rare case and literature. Curr Pharm Biotechnol 14: 859-863.
14. Zhu G, Saboor-Yaraghi AA, Yarden Y, Santos J, Neil JC (2016) Down regulating oncogenic receptor: From bench to clinic. Hematol Med Oncol 1: 30-40.
15. Zhu G, Saboor-Yaraghi AA, Yarden Y (2017) Targeting oncogenic receptor: From molecular physiology to the currently the standard of target therapy. Adv Pharm J 2: 10-28.
16. Green S, Chambon P (1986) Carcinogenesis: A superfamily of potentially oncogenic hormone receptors. Nature 224: 615-617.
17. Singh RR, Kumar R (2005) Steroid hormone receptor signaling in tumorigenesis. J Cell Biochem 95: 250-260.
18. Montgomery RR, Ducore JM, Githens JH, August CS, Johnson ML (1980) Regression of oxymetholone-induced hepatic tumors after bone marrow transplantation in aplastic anemia. Transplantation 30: 90-95.
19. Kemp CT (1989) Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci U S A 86: 9707-9709.
20. Castells A, Bruix J, Bru C (1995) Treatment of hepatocellular carcinoma with tamoxifen: A double-blind placebo-controlled trial in 120 patients. Gastroenterology 109: 917-922.
21. Chow PK, Tai BC, Tan CK (2002) High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology 36: 1221-1226.
22. Konda H, Tajima H, Lee GH, Homura K, Ohtake K, et al. (1992) Hepatocyte growth factor transformed immortalized mouse liver epithelial cells. Oncogene, 8: 3047-3053.
23. Gabitova L, Gorin A, Astsaturov I (2013) Molecular pathways: Sterols and receptor signaling in cancer. Clin Cancer Res 19: 6344-6350.
24. Porter AC, Vaillancourt RR (1998) Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene 17: 1343-1352.
25. Reilly JT (2002) Class III receptor tyrosine kinases: Role in leukemogenesis. Br J Haematol 116: 744-757.
26. Verstraete K, Savvides SN (2012) Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat Rev Cancer 12: 753-766.
27. Kirschbaum MH, Marmor MD, Yarden Y (2003) Oncogenic receptor tyrosine kinases. Cancer Drug Discov Dev 47: 75.
28. Mizuki M (2003) Oncogenic receptor tyrosine kinase in leukemia. Cell Mol Biol 49: 907-922.
29. Blume-Jensen P, Hunter T (2001) Oncogenic kinase signaling. Nature 411: 355-365.
30. Zhu G, Zhu QW (2017) A powder preparation of human epidermal growth factor (hEGF), 5% Fu of EGF ointment and cosmetics hEGF face-cream and EGF bath cream (dew). China Patent Application No: 201710144050.0
31. Abou-Alfa GK, Schwartz L, Ricci S (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 4293-4300.
32. Abou-Alfa GK (2009) Selection of patients with hepatocellular carcinoma for sorafenib. J Nat Compr Cancer Netw 4: 397-403.
33. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390.
34. Cheng AL, Kang YK, Chen ZD, Tsao CJ, Qin SK, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34.
35. Yau T, Chan P, Ng KK, Chok SH, Cheung TT, et al. (2009) Phase 2 open-label study of single agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: Presence of lung metastasis predicts poor response. Cancer 115: 428-436.
36. Goyal L, Muzamdar MD, Zhu AX (2013) Targeting the HGF/c-Met pathway in hepatocellular carcinoma. Clin Cancer Res 9: 2310-2318.
37. Vigna E, Chiriaco C, Cignetto S, Fontanini L, Basilico C, et al. (2016) Inhibition of ligand-independent constitutive activation of the Met oncogenic receptor by the engineered chemically-modified antibody DN30. Mol Oncol 9: 1760-1772.
38. Vigna E, Pacchiana G, Chiria C, Cignetto S, Fontani L, et al. (2014) Targeted therapy by gene transfer of monovalent antibody fragment the Met oncogenic receptor. J Mol Med (Berl) 92: 65-76.
39. Zhu G, Musumecci F, Byrne P, Gupta D, Gupta E (2017) Targeting oncogenic receptor, currently the standard of care. Clin Trials Pathol Case Stud 2: 75-90.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Pathology and Toxicology Research
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)