1123
Views & Citations123
Likes & Shares
ACI: Autologous Chondrocyte Implantation; hESC: Human Embryonic Stem Cell; MMP:
Matrix Metalloprotease; iPSC: Induced Pluripotent Stem Cell; MSC: Mesenchymal
Stem Cell; PGE2: Prostaglandin E2; TGF: Transforming
Growth Factor; MIA: Melanoma Inhibitory Activity.
Osteoarthritis
(OA) is a degenerative disease of cartilage that is common in elderly people.
OA becomes progressively worse, and late-stage OA patients have no choice but
to undertake total knee arthroplasty as a radical cure. This paper reviews the
current conventional medical treatments and novel therapies aimed at inducing
cartilage regeneration. Transplantation of layered chondrocyte sheets is a
promising novel option for patients with cartilage lesions including OA.
Layered chondrocyte sheets have been shown to exhibit a cartilage-restoring
effect in experimental animal models of cartilage defects. The safety and
efficacy have been examined in humans. This review discusses the mode of action
of cell sheets in cartilage restoration and future prospects.
INTRODUCTION
Articular cartilage bears the body’s weight and may wear away as a result
of daily activities. The main components of articular cartilage are water,
which comprises 70%-80% of the total weight, collagen
(50%-70% of the dry weight), and proteoglycan
(~30% of the dry weight). Articular chondrocytes maintain hyaline cartilage by
producing extracellular matrix, which comprises collagens, proteoglycans, and
enzymes essential for cartilage tissue metabolism. However, articular
chondrocytes comprise less than 5% of articular cartilage tissue by volume [1].
Because of the absence of blood vessels and low density of chondrocytes,
damaged cartilage can be only minimally repaired, especially in elderly
patients.
Osteoarthritis (OA) affects 30%-50% of people aged 65 years or
older and is considered to be a degenerative disease of cartilage [2].
Overweight, obesity, female gender, and knee injury are recognized risk factors
for OA. The onset of OA is associated with previous joint injury in 5% of cases
and with weight gain or obesity in 25% of cases [3]. Body weight management is
an effective intervention to prevent or slow disease progression. Restoration
of damaged cartilage should be considered from the early stage of OA.
Joint injury eventually causes OA. Malalignment of bones and joint
instability cause inappropriate load-bearing contact in the joint, which causes
the articular cartilage to wear out [4]. Injury to knee cartilage causes
gradual loss of the extracellular matrix and disruption of the cartilage
structure, which lead to subchondral bone exposure and the onset of knee pain.
The changing microenvironment disrupts chondrocyte function and worsens the
cartilage defect.
Late OA patients often receive total knee arthroplasty (TKA). Ninety-three percent of patients are generally satisfied 5 years postoperatively; 87% are satisfied with the relief of pain and 80% are satisfied with the improvement in physical function at that time.
However, patients’ preoperative expectations may be higher than their
postoperative ability to undertake leisure activity and walking [5].
Novel therapeutic applications for the treatment of OA are needed to
meet patients’ expectations of medical treatment and postoperative daily life.
Conventional Regenerative Medicine for
Cartilage Damage
Joint trauma and osteochondritis dissecans are other pathological
conditions that can cause cartilage damage. Surgical interventions aim to
reestablish the joint surface. The choice of the surgical procedure is based on
the size of the damaged area, joint stability, and the patient’s age and
symptoms.
Microfracture is one procedure performed to stimulate the damaged
cartilage to fill with tissue made by migrating mesenchymal stem cells (MSCs)
derived from the bone marrow [6,7]. However, the repaired cartilage exhibits
characteristics of fibrous cartilage and not hyaline cartilage, and the
procedure has poor clinical outcomes on a long period of time [8].
Autologous osteochondral mosaicplasty can be applied to small and
medium-sized osteochondral lesions. The cartilaginous surface is reconstructed
using osteochondral grafts obtained from autologous non weight-bearing
cartilaginous parts. Grafts provide a hyaline cartilage surface, but the
intergraft spaces tend to be filled with fibrous cartilage [9-11].
First reported by Brittberg et al. [12], autologous chondrocyte
implantation (ACI) is now the most commonly used cell-based therapy for the
treatment of cartilage defects in young patients and has been applied to over
20,000 patients worldwide [13]. Lynch et al. [14] reported superior clinical
results of mosaicplasty compared with microfracture. They reported a higher
rate of return to sport and maintenance of patients’ sports ablity after
surgery, and a lower rate of reoperation. Compared with ACI, the prognostic
superiority of mosaicplasty is not conclusive, and mosaicplasty has a higher
failure rate. A high incidence (49%) of a subsequent surgical procedure has
been reported [15]. The cartilage tissue morphology generated after ACI had
been found to be predominantly hyaline in 22% of biopsy specimens, mixed in
48%, and predominantly fibrocartilage in 30% [16]. Because hyaline cartilage
restoration is very important to joint function, the effects of ACI [17,18] and
the outcomes of all available therapies for damaged cartilage are insufficient.
In addition, the effectiveness of these therapies in treating damaged cartilage
associated with OA has not been confirmed, and thus there is no authorized
treatment for cartilage restoration in OA patients. To address these issues, a
novel therapy using cell sheet technology to treat damaged cartilage has been
developed.
Cartilage Regeneration Using Cell Sheets
Cell sheet technologies have been applied to many cell types and
therapeutic applications [19] including the cornea [20], esophagus [21],
myocardium [22], and periodontium [23]. Kaneshiro et al. [24] introduced cell
sheet technologies in the treatment of cartilage regeneration. Cell sheets can
be created using poly (N-isopropylacrylamide), a thermoresponsive polymer and
grafting in a culture dish [25,26]. The thermoresponsive surface of the culture
dish allows for the noninvasive harvesting of intact sheets of cells within
their deposited extracellular matrix. Using this approach, cell sheets can be
transplanted into host tissues without the use of scaffolding or carrier
materials [27].
Chondrocytes can adhere to and proliferate on the thermoresponsive
polymer-grafted plate surface. When cells become confluent, they produce
chondrogenic extracellular matrix and the cell sheets become thick. Chondrocyte
sheets can be readily detached from these surfaces by lowering the incubation
temperature without the need for enzymes to digest the extracellular matrix.
Incorporating the cells within the extracellular matrix allows the chondrocytes
in the cell sheet to retain their adherent molecules, receptors, cell-cell
contact, and tissue microenvironment.
Multilayered chondrocyte sheets can be created by simply stacking three
cell sheets and cultivating them for 1 additional week. The triple-layered
chondrocyte sheets provide a fused monolithic structure with sufficient
strength to be transplanted [28].
Transplantation of layered chondrocyte sheets onto a partial-thickness
defect created in the knee cartilage of Japanese white rabbit prevented
cartilage tissue degeneration [24]. In a rabbit total-thickness defect model,
layered chondrocyte sheets seemed to alleviate pain and stimulate tissue
repair. Sheet transplantation has produced excellent results for both
defect-filling rates and subchondral bone formation. The graft cartilage layer
exhibits a columnar arrangement showing repair with hyaline cartilage [29].
Cartilage restoration has also been reported for layered chondrocyte sheets
applied to full-thickness cartilage defects in a minipig model [30]. The
cartilage-regenerating effects achieved with cell sheets were the same as those
achieved with tissue-engineered cartilage with a scaffold [31,32] or scaffold
less cartilage discs [33,34].
The pathogenesis of OA includes a mix of full- and partial-thickness
cartilage defects. Generally, partial-thickness cartilage defects are more
difficult to restore because of the lack of chondrogenic progenitor cells.
Layered chondrocyte sheets can induce cartilage-restoring effects in both
partial-and total-thickness defect models, as mentioned above. This suggests
that the sheets may be effective in treating cartilage lesions caused by OA.
Human articular chondrocytes have low proliferative capacity. The poor
availability and yield of cells from patients limit the development of feasible
therapies. Because coculture with synovial cells promotes the proliferation of
human articular chondrocytes,to overcome this difficulty, human articular
chondrocytes are cocultured with synovial cells to create human layered
chondrocyte sheets [35].
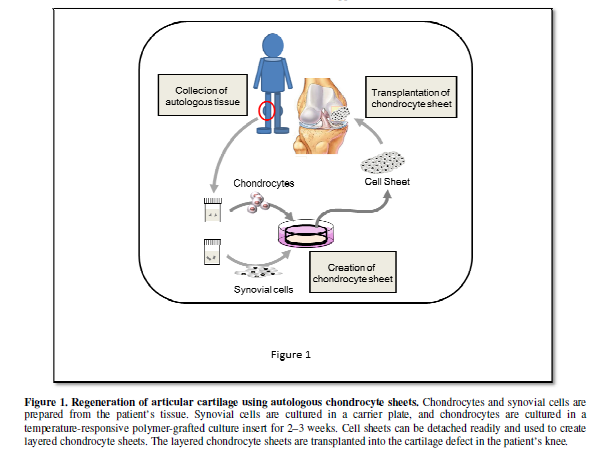
Based on these encouraging results in experimental cartilage defect models and the establishment of cell sheet preparation procedures, a clinical study of the transplantation of human layered chondrocyte sheets into cartilage defects, including those caused by OA, has been conducted and completed safely. This study has shown the efficacy of this procedure (Figure 1). A manuscript is in preparation and the results will appear elsewhere.
Mode of Action of Chondrocyte Sheets in
Cartilage Regeneration
Triple-layered chondrocyte sheets express genes that are critical to
cartilage maintenance, including those encoding type II collagen, aggrecan-1,
and tissue metallopeptidase inhibitor 1, but not those encoding type I
collagen, matrix metalloproteinase (MMP)-3, MMP-13, and A-disintegrin and
metalloproteinase with thrombospondin motifs 5 [35]. Expression of the gene
encoding the adhesion factor fibronectin-1 has also been reported [35]. Mitani
et al. [28] reported the increased
expression of SOX9, collagen type 27, and integrin alpha 10 in triple-layered
chondrocyte sheets compared with monolayer cultures. This finding suggests that
the layered structure contributes to the maintenance of the cartilaginous
characteristics.
Hamahashi et al. [36]
evaluated the secretion of humoral factors by layered chondrocyte sheets.
Production of collagen type 1, collagen type 2, MMP-13, transforming growth
factor-b (TGFb), melanoma inhibitory activity
(MIA), and prostaglandin E2 (PGE2) were detected by
enzyme-linked immunosorbent assays. Higher concentrations of PGE2
and TGFb were detected in the supernatants from cell
sheets compared with those from ordinary cell cultures.
MIA is recognized as a marker of chondrocytes. MIA and collagen type II
mRNA expression correlates specifically with chondrogenic differentiation and
is not induced by osteoblastic differentiation [37]. By modulating the actions
of bone morphogenetic protein-2 and TGFb3 during mesenchymal stem cell
differentiation, MIA supports the chondrogenic phenotype while inhibiting
osteogenic differentiation [38]. Nishitani et al. [39] demonstrated that PGE2 inhibits IL-1b-induced
MMP-1 and MMP-13 production via prostaglandin E receptor 4 by suppressing the
mitogen-activated protein kinase - Jun N terminal kinase pathway.
These results suggest that the humoral factors produced by layered
chondrocyte sheets may contribute to cartilaginous tissue repair. Kaneshiro et
al. [40] demonstrated that layered
chondrocyte sheets adhered firmly to porcine cartilage after 1 day of culture.
Histological analysis showed reduced safranin-O staining intensity of partially
damaged cartilage tissue, whereas good staining intensity was observed in the
damaged tissue covered by the layered cell sheet. This finding suggests that
leakage of proteoglycans and cartilage degeneration occur in partial cartilage
defects and that layered chondrocyte sheets can prevent these effects.
Another hypothesis is that cell sheets may provide chondrogenic
progenitor cells for cartilage regeneration at the transplanted site. To investigate
the cell fate in recipient animals, Takaku et al. [41] established a method for tracking cell sheets noninvasively
and consecutively using luciferase-expressing chondrocyte sheets created from
transgenic Lewis rats. The luciferase-expressing chondrocytes were monitored
continuously using bioluminescence imaging. They found that the transplanted
cells remained in the joint after 21 months and did not migrate to other parts
of the body. However, the intensity of the luciferase signal decreased rapidly
after transplantation, which suggests that the transplanted sheets were less
likely to act as the main source of chondrocytes in the restored cartilage
tissue.
Taken together, these findings suggest that chondrocyte sheets can
contribute to cartilage regeneration by providing anabolic factors for
chondrogenesis, by protecting against catabolic factors in the joint cavity,
and by preventing loss of the extracellular matrix.
Future Cell Sources for Cell Sheet Technology
Cell sourcing is one obstacle to the development and clinical
application of regenerative therapy using cell sheets. The proliferative
capacity and characteristics of autologous cells can vary, which may affect the
reliability of cell sheet therapy and clinical outcomes. Patients must
undertake two surgical procedures—one to collect autologous tissue and a second
to transplant the cell sheets. Other cell sources have been explored to
overcome these problems.
Cartilage is considered an immune-privileged tissue, and allogeneic
cartilage tissue is now used as a cell source. Allogenic juvenile articular
cartilage grafts (DeNovo® NT Natural Tissue Graft; Zimmer, Warsaw,
IN) have been used in more than 7500 patients with cartilage defects. Because
primary adult chondrocytes have limited proliferative capacity and their
long-term cultivation causes dedifferentiation [42], stem cells are considered
as a possible source of chondrocyte progenitor cells.
Human embryonic stem cells (hESCs) and induced pluripotent stem cells
(iPSCs) are reasonable candidates as a cell source. These cells have infinite
proliferative capacity and can provide enough cells for therapeutic
applications. However, the use of hESCs raises ethical concerns. Theoretically,
iPSCs can be established from any individual. Considering the immune-privileged
characteristics of cartilage, certain iPSC cell lines may be applicable to all
patients. However, iPSCs require multistep, long-term procedures to obtain
properly differentiated chondrocytes or chondrogenic progenitor cells [43,44].
Another concern relating to the risks associated with the tumorigenic potential
of iPSCs needs to be addressed [45].
Multipotent MSCs exhibit potential for chondrogenic differentiation and
have been found in various tissues such as bone marrow, synovial tissue,
adipose tissue, umbilical cord, and skin. Many procedures for chondrogenic
differentiation of MSCs have been reported [46]. Except for umbilical cord
MSCs, these cells can be prepared from individual patients. Allogeneic MSCs may
also be applicable. However MSCs have a finite proliferative capacity.
The possible methods for preparing the cell source for cartilage
regeneration using cell sheet technology need further evaluation. The safety,
characteristics of the chondrocytes obtained, and costs of preparation must
also be considered.
CONCLUSION
Restoration of damaged cartilage using chondrocyte sheets is a
promising novel regenerative therapy for OA or cartilage lesions. The use of
allogeneic chondrocytes as a cell source for chondrocyte sheets needs further
evaluation before this therapy can be offered as standard treatment. The
multistep, long-term procedure required for preparation of chondrocyte sheets
directly affects the feasibility of regenerative therapy. The need for quality
differentiated cells and the establishment of feasible procedures will
determine which cell sources are used in this technology.
ACKNOWLEDGEMENT
The study was
supported by a grant from a Health Labour Sciences Research Grant (12103253 to
Masato Sato) from the Ministry of Health, Labour, and Welfare of Japan. The
authors have no conflicts of interest to declare.
- Little
CJ, Bawolin NK, Chen X (2011) Mechanical properties of natural cartilage
and tissue-engineered constructs. Tissue Eng Part B Rev 17: 213-227
- Loeser
RF (2010) Age-related changes in the musculoskeletal system and the
development of osteoarthritis. Clin Geriatr Med 26: 371-386.
- Silverwood
V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, et al. (2015)
Current evidence on risk factors for knee osteoarthritis in older adults:
a systematic review and meta-analysis. Osteoarthritis Cartilage 23:
507-515.
- Andriacchi
TP, Mündermann A (2006) The role of ambulatory mechanics in the initiation
and progression of knee osteoarthritis. Curr Opin Rheumatol 18: 514-518.
- Nilsdotter
AK, Toksvig-Larsen S, Roos EM (2009) Knee arthroplasty: are patients’
expectations fulfilled? A prospective study of pain and function in 102
patients with 5-year follow-up. Acta Orthop 80: 55-61.
- Steadman
JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness
chondral defects: surgical technique, rehabilitation, and outcomes. J Knee
Surg 15: 170-176.
- Mithoefer
K, Williams RJ, Warren RF, Potter HG, Spock CR, et al. (2006) Chondral
resurfacing of articular cartilage defects in the knee with the
microfracture technique. Surgical technique. J Bone Joint Surg Am 2:
294-304.
- Buckwalter
JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis,
repair, regeneration, and transplantation. Instr Course Lect 47: 487-504.
- Hangody
L, Kish G, Kárpáti Z, Udvarhelyi I, Szigeti I, et al. (1998) Mosaicplasty
for the treatment of articular cartilage defects: application in clinical
practice. Orthopedics 21: 751-756.
- Szerb
I, Hangody L, Duska Z, Kaposi NP (2005) Mosaicplasty: long-term follow-up.
Bull Hosp Jt Dis 63: 54-62.
- Robert
H (2011) Chondral repair of the knee joint using mosaicplasty. Orthop
Traumatol Surg Res 97: 418-429.
- Brittberg
M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, et al. (1994) Treatment of
deep cartilage defects in the knee with autologous chondrocyte
transplantation. N Engl J Med 331: 889-895.
- Peterson
L, Minas T, Brittberg M, Lindahl A (2003) Treatment of osteochondritis
dissecans of the knee with autologous chondrocyte transplantation: results
at two to ten years. J Bone Joint Surg Am 85-A Suppl 217-224.
- Lynch
TS, Patel RM, Benedick A, Amin NH, Jones MH, et al. (2015) Systematic
review of autogenous osteochondral transplant outcomes. Arthroscopy 31:
746-754.
- Zaslav
K, Cole B, Brewster R, DeBerardino T, Farr J, et al. (2009) A prospective
study of autologous chondrocyte implantation in patients with failed prior
treatment for articular cartilage defect of the knee: results of the Study
of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports
Med 37: 42-55.
- Roberts
S, McCall IW, Darby AJ, Menage J, Evans H, et al. (2003) Autologous
chondrocyte implantation for cartilage repair: monitoring its success by
magnetic resonance imaging and histology. Arthritis Res Ther 5: R60-R73.
- Wood
JJ, Malek MA, Frassica FJ, Polder JA, Mohan AK, et al. (2006) Autologous
cultured chondrocytes: adverse events reported to the United States Food
and Drug Administration. J Bone Joint Surg Am 88: 503-507.
- Nawaz
SZ, Bentley G, Briggs TWR, Carrington RWJ, Skinner JA, et al. (2014)
Autologous chondrocyte implantation in the knee: mid-term to long-term results.
J Bone Joint Surg Am 96: 824-830.
- Owaki
T, Shimizu T, Yamato M, Okano T (2014) Cell sheet engineering for
regenerative medicine: current challenges and strategies. Biotechnol J 9:
904-914.
- Nishida
K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, et al. (2004) Corneal
reconstruction with tissue-engineered cell sheets composed of autologous
oral mucosal epithelium. N Engl J Med 351: 1187-1196.
- Ohki
T, Yamato M, Ota M, Takagi R, Murakami D, et al. (2012) Prevention of
esophageal stricture after endoscopic submucosal dissection using
tissue-engineered cell sheets. Gastroenterology 143: 582-588.
- Sawa
Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, et al. (2012) Tissue
engineered myoblast sheets improved cardiac function sufficiently to
discontinue LVAS in a patient with DCM: report of a case. Surg Today 42:
181-184.
- Iwata
T, Washio K, Yoshida T, Ishikawa I, Ando T, et al. (2015) Cell sheet
engineering and its application for periodontal regeneration. J Tissue Eng
Regen Med 9: 343-356.
- Kaneshiro
N, Sato M, Ishihara M, Mitani G, Sakai H, et al. (2006) Bioengineered
chondrocyte sheets may be potentially useful for the treatment of partial
thickness defects of articular cartilage. Biochem Biophys Res Commun 349:
723-731.
- Okano
T, Yamada N, Okuhara M, Sakai H, Sakurai Y (1995) Mechanism of cell
detachment from temperature-modulated, hydrophilic-hydrophobic polymer
surfaces. Biomaterials 16: 297-303.
- Okano
T, Yamada N, Sakai H, Sakurai Y (1993) A novel recovery system for
cultured cells using plasma-treated polystyrene dishes grafted with
poly(N-isopropylacrylamide). J Biomed Mater Res 27: 1243-1251.
- Yang
J, Yamato M, Nishida K, Ohki T, Kanzaki M, et al. (2006) Cell delivery in
regenerative medicine: the cell sheet engineering approach. J Control Release
116: 193-203.
- Mitani
G, Sato M, Lee JIK, Kaneshiro N, Ishihara M, et al. (2009) The properties
of bioengineered chondrocyte sheets for cartilage regeneration. BMC
Biotechnol 9: 17.
- Ito
S, Sato M, Yamato M, Mitani G, Kutsuna T, et al. (2012) Repair of
articular cartilage defect with layered chondrocyte sheets and cultured
synovial cells. Biomaterials 33: 5278-5286.
- Ebihara
G, Sato M, Yamato M, Mitani G, Kutsuna T, et al. (2012) Cartilage repair
in transplanted scaffold-free chondrocyte sheets using a minipig model.
Biomaterials 33: 3846-3851.
- Masuoka
K, Asazuma T, Ishihara M, Sato M, Hattori H, et al. (2005) Tissue
engineering of articular cartilage using an allograft of cultured
chondrocytes in a membrane-sealed atelocollagen honeycomb-shaped scaffold
(ACHMS scaffold). J Biomed Mater Res B Appl Biomater 75: 177-184.
- Masuoka
K, Asazuma T, Hattori H, Yoshihara Y, Sato M, et al. (2006) Tissue
engineering of articular cartilage with autologous cultured adipose
tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold
with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater 79:
25-34.
- Nagai
T, Sato M, Furukawa KS, Kutsuna T, Ohta N, et al. (2008) Optimization of
allograft implantation using scaffold-free chondrocyte plates. Tissue Eng
Part A 14: 1225-1235.
- Nagai
T, Furukawa KS, Sato M, Ushida T, Mochida J (2008) Characteristics of a
scaffold-free articular chondrocyte plate grown in rotational culture.
Tissue Eng Part A 14: 1183-1193.
- Kokubo
M, Sato M, Yamato M, Mitani G, Kutsuna T, et al. (2013) Characterization
of chondrocyte sheets prepared using a co-culture method with
temperature-responsive culture inserts. J Tissue Eng Regen Med. Doi:
10.1002/term.1764
- Hamahashi
K, Sato M, Yamato M, Kokubo M, Mitani G, et al. (2015) Studies of the
humoral factors produced by layered chondrocyte sheets. J Tissue Eng Regen
Med 9: 24-30.
- Bosserhoff
AK, Buettner R (2003) Establishing the protein MIA (melanoma inhibitory
activity) as a marker for chondrocyte differentiation. Biomaterials 24:
3229-3234.
- Tscheudschilsuren
G, Bosserhoff AK, Schlegel J, Vollmer D, Anton A, et al. (2006) Regulation
of mesenchymal stem cell and chondrocyte differentiation by MIA. Exp Cell
Res 312: 63-72.
- Nishitani
K, Ito H, Hiramitsu T, Tsutsumi R, Tanida S, et al. (2010) PGE2 inhibits
MMP expression by suppressing MKK4-JNK MAP kinase-c-JUN pathway via EP4 in
human articular chondrocytes. J Cell Biochem 109: 425-433.
- Kaneshiro
N, Sato M, Ishihara M, Mitani G, Sakai, H, et al. (2007) Cultured
articular chondrocytes sheets for partial thickness cartilage defects
utilizing temperature-responsive culture dishes. Eur Cell Mater 13: 87-92.
- Takaku
Y, Murai K, Ukai T, Ito S, Kokubo M, et al. (2014) In vivo cell
tracking by bioluminescence imaging after transplantation of bioengineered
cell sheets to the knee joint. Biomaterials 35: 2199-2206.
- Darling
EM, Athanasiou KA (2005) Rapid phenotypic changes in passaged articular
chondrocyte subpopulations. J Orthop Res 23: 425-432.
- Yamashita
A, Liu S, Woltjen K, Thomas B, Meng G, et al. (2013) Cartilage tissue
engineering identifies abnormal human induced pluripotent stem cells. Sci
Rep 3: 1978.
- Yamashita
A, Morioka M, Yahara Y, Okada M, Kobayashi T, et al. (2015) Generation of
scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep
4: 404-418.
- Kamada
M, Mitsui Y, Kumazaki T, Kawahara Y, Matsuo T, et al. (2014) Tumorigenic
risk of human induced pluripotent stem cell explants cultured on mouse
SNL76/7 feeder cells. Biochem Biophys Res Commun 45: 668-673.
- Lee
JK, Responte DJ, Cissell DD, Hu JC, Nolta JA, et al. (2014) Clinical
translation of stem cells: insight for cartilage therapies. Crit Rev
Biotechnol 34: 89-100.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Alcoholism Clinical Research
- Journal of Spine Diseases
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)