1728
Views & Citations728
Likes & Shares
This study was designed for analyzing the current concepts about GH and
stem cells treatments in some acquired neurological injuries (cerebral palsy,
stroke, traumatic brain injury) and myocardial infarction. From this analysis
we can conclude that while it seems that GH plays an important therapeutic
role, it is also clear that stem cells are a promising therapeutic alternative;
althoughthere is still a need to clarify what is the optimal window of time for
their administration after each one of these damages, as well as the best route
of administration in each case and the most appropriate number of stem cells
that should be administered. Since the largest number of implanted stem cells
do not integrate into the damaged tissue, but rather exert their actions by
releasing a number of trophic factors, most of them physiologically induced by
GH, and die within a few days after being administered, studies must be done to
try to genetically modify these stem cells in GMP facilities so that they can
replace and repair the damaged tissues. Here we also provide evidences
indicating that GH administration may be of utility for increasing the number
of endogenous, and exogenously administered, stem cells allowing their
survival, differentiation and migration to the damaged area. In adition, we
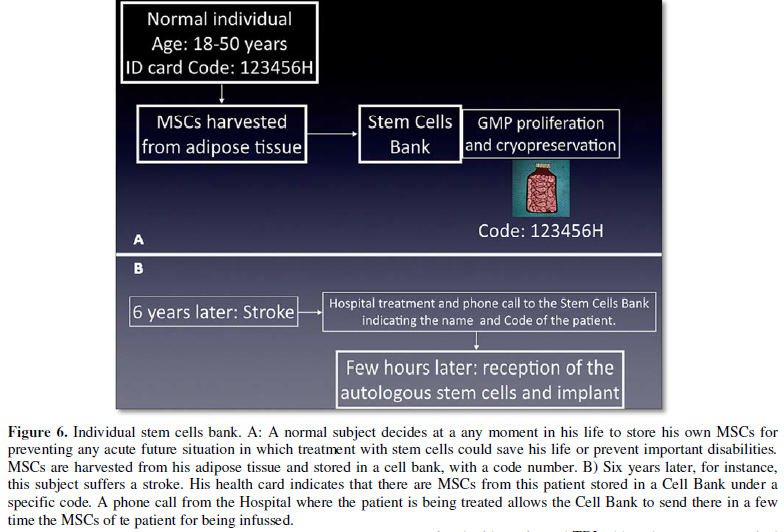
suggest that each individual may have their stem cells stored in a cell bank,
so that he could receive them early after any of these injuries.
Keywords: Growth hormone,
Stem cells, Cerebral palsy, Stroke, Traumatic brain injury, Cardiac infarction
Abbreviations: GH: Growth Hormone; IPSCs: Induced
Pluripotent Stem Cells; HSCs: Hematopoietic Stem Cells; MSCs: Mesenchymal Stem
Cells; NSCs: Neural Stem Cells; GMP: Good Manufacturing Practice; TBI:
Traumatic Brain Injury; CSCs: Cardiac Stem Cells; EPCs: Endothelial Progenitor
Cells
INTRODUCTION
In this review we will analyze whether the administration of Growth
hormone, concomitantly with the implants of stem cells and/or subsequently to
these, could improve the results of stem cells therapies in four pathologies
(cerebral palsy, stroke, traumatic brain injury and myocardial infarction),
that due to their high prevalence, morbidity and mortality require the use of
new therapeutic strategies.
We first analyze the therapeutic effects of GH, given alone, and then the
current knowledge about stem cells therapies. Lastly we will consider whether
GH might be useful for being administered conjointly with stem cells for
improving the effects of these therapies.
Growth Hormone (GH)
Classically GH has been considered a metabolic hormone, produced by the pituitary
gland, responsible for the longitudinal growth of the organism until the end of
puberty. However, this quite simple concept has been changed in the last years
because of a number of findings indicating that the hormone plays in the human
body quite more and different important effects far beyond than those
previously established [1]. Moreover, apart of its well known pituitary
production and release for playing an endocrine role, we know since years ago,
that both the hormone and its receptor (GHR) are expressed in practically all
tissues, if not in all of them, in which the hormone plays an auto/paracrine
role [2-5].
Particularly important is the expression of GH, and its receptor, in neural
stem cells where it seems to play a key role in the proliferation,
differentiation, migration and survival of these neural precursors [6,7], therefore
suggesting that GH might be an important factor for brain repair after an
injury, a hypothesis already proposed many years ago [8]. Apart of the crucial
role that the GH/IGF-I system plays during the brain development in embryos [9,10],
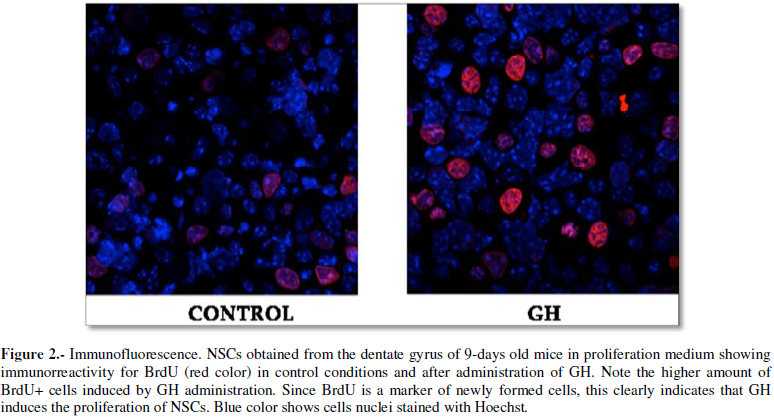
the expression of GH in neurogenic regions of the postnatal brain, as Figure 1 shows, has been demonstrated
in a number of already ancient studies [11,12]. These and many other studies
led to the possibility that GH administration could be of utility, in humans
and some laboratory animals, in the repair of brain after an injury (cerebral
palsy, stroke or traumatic brain injury), a possibility already demonstrated by
a number of studies from ours and other groups [13-34]. In fact, in rats, the
expression of GH and its receptor is markedly upregulated after brain injury,
suggesting that the hormone may enhance neuroregeneration after brain injury
[35]. However, the positive effects of GH administration on brain repair,
inducing the proliferation on stem cells existing in neurogenic niches of the
brain (Figure 2), could only be
observed in laboratory animals in which brain injury had previously been
induced [26,35,36] or in studies in vitro
[28,37,38].
GH
and cardiovascular system
GH plays also very important effects on the cardiovascular system. It has
been shown that an endothelial dysfunction exists in GH-deficient (GHD)
patients leading to a lesser edothelium-dependent vasodilation [39]. This is
most likely produced by a lesser nitric oxide (NO) production by the
endothelium [40]. The administration of GH restores the affected endothelial
function and increases the production of NO [39-41], and decreases the
oxidative stress associated to the endothelial damage [39]. Moreover, GH
administration is able to reverse the intima-media thickness [5,39]. From this
and other data it is likely that GH can play a very important role as an
inductor of the mechanisms physiologically involved in recovery after a
cardiovascular injury. Recent pre-clinical studies support such a possibility.
For instance, it has been proven, in rats, that treatment with GH after myocardial
infarction enhanced angiogenesis and myofibroblast activation and improves
post-infarction remodeling [42]. This was also observed in another study in
which GH was delivered from an alginate scaffold injected around the ischemic
area of myocardium after coronary ligation, leading to a clear amelioration of
ventricular function and exhibiting long-term antiarrhytmic potential [43].
Stem cells
Since the late 70's, stem cells therapies have been a promising strategy
for the treatment of many diseases, including cancer, AIDS, heart infarctions,
stroke, traumatic brain injuries, lung affectations, cerebral palsy, and a
number of neurodegenerative and eye diseases, blood diseases, liver failure,
diabetes, colitis, cartilage degenerations, among many others.
Stem cell therapies had their origin in older therapies, such as blood
transfusion, bone marrow and organ transplantation and in vitro fertilization. Improvement of these therapies soon started
to require ex vivo processing before
their use as a pharmaceutical product, moving from "transplantation"
to "stem cell therapy".
There are many potential forms of stem cells therapies depending on the
finalaim of the therapeutic strategy. In general we can envisage three main
different aims when a stem cell therapy strategy is designed:
1.
To restore the cell
population that has been lost or damaged. These include regeneration of blood
vessels, brain, heart, liver, cartilage and bone.
2.
To modify immune
responses to either enhance (anti-tumor) or to lower (autoimmune diseases) T
and B cell responses.
3.
To restore normal
functions of tissues or cells through paracrine secretions of soluble factors.
To date, according to the information provided by the U.S. National Library
of Medicine (http://www.clinicaltrials.gov, November 2017),
there are 4565 studies for stem cells therapies in a really high number of different
diseases, including a rare form of Parkinsonism as it is the Progressive
Supranuclear Palsy.
The different cell types used in the treatments currently carried out can
come from the same patient (autologous) or can be derived from a donor
(allogeneic). Depending on the country and its specific health laws, the origin
of the cells used may be quite different; for instance, pluripotent stem cells
(human embryonic stem cells or induced pluripotent stem cells (IPSCs) [44] and
even stem cells obtained from parthenogenetically activated human oocytes [45])
are not allowed for its use in stem cells therapies in Europe but in other
ountries, such as China and USA. However, due to ethical reasons and/or
technical difficulties together with the possibility of inducing important
adverse side effects (i.e., tumorigenicity in the case of human embryonic stem
cells, and perhaps IPSCs too), during the last years the field of stem cells
therapies is progressing towards the clinical use of multipotent adult stem
cells obtained from many different sources. These adult stem cells are found in
all adult tissues and they physiologically participate in the regeneration of
the tissues where they belong. This is the case, for example, of hematopoietic
stem cells (HSCs), mesenchymal stem cells (MSCs) and neural stem cells (NSCs).
HSCs
HSCs are able to migrate to the bone marrow and give rise to all
hematopoietic cell types when injected intravenously; therefore they are useful
for treating primary immunodeficiencies and hemoglobinopaties, but also for
treating cancer, neurodegenerative and cardiac disorders. Of course these
treatments would be ideally performed by using autologous cells, but HSCs
(originally characterised in humans as CD34+, Lin- cells) from healthy
HLA-compatible donors orgene-modified allogeneic HSCs could also be used. This
would impede the appearance of graft versus host disease.
CD34 is a cell surface antigen, first discovered in a cell surface
glycoprotein [46,47] that is early expressed in hematopoietic and
vascular-associated tissue [48]. It is an adhesion molecule, but also
facilitates cell migration [48], including chemokine-dependent migration of
eosinophils, hence facilitatingthe development of allergic asthma [49].
Moreover, HSCS don't express mature blood cells markers, such as Lin
(lineage-positive cells). Therefore, HSCs can be easily identified and isolated
by flow cytometry from other blood cells in the sample, by staining them with
specific markers for CD34 and Lin.
MSCS
MSCs are very attractive stem cells for use in
clinic because: 1) they are able to give rise to different tissue types and therefore could, at least theoretically, be used for tissue repair; 2) they
are able to modulate the immune system and can therefore be used as a way to
decrease immune responses; 3) MSCs are quite easy to be isolated and expanded.
MSCs can be obtained from many different tissues, such as: bone marrow,
adipose tissue, dental pulp, synovial membranes, Wharton's jelly, umbilical
cord blood, liver tissue, etc. They have to be positive for CD105, CD90 and
CD73, negative for MHC-II, CD11b, CD14, CD31, CD34 and CD45 and also express
low levels of MHC-I.
MSCs have shown important therapeutic benefits in several pathologies
including graft versus host disease, diabetes, cardiovascular diseases, bone
and cartilage diseases, neurological diseases, liver and lung diseases, Crohn
disease [50] and recently they have been proved to be useful in spinal cord
injuries [51]. In addition, the ability of MSCs to differentiate into
epitelia-like cells have suggested that they could be useful to contribute to
wound repair when administered locally [52-55]. Moreover, a special type of
MSCs, the Muse cells (Multilineage-differentiating stress-enduring) can be
obtained from cultured bone marrow-mesenchymal stem cells showing important
repair effects after being transplanted into mice with neurological diseases
because they differentiate into neurons and connect with host intact neurons
[56].
NSCs
NSCs transplantation has been studied in animal models as an attempt to
repair brain and spinal cord injuries. However, of all the possible neural
sources of NSCs only the olfactory ensheathing cells, obtained from the
olfactory mucosa, are readily accessible and capable of lifelong regeneration.
They have been used in laboratory animals with spinal cord injuries, but it is
still not clear that these cells will produce significant positive effects in
humans with similar spinal cord injuries. However, a recent work in rats
demonstrated that NSCs expanded from the postnatal subventricular zone
engrafted into the hippocampus of young and aged animals leading to the
production of newly born neurons and even to the appearance of new neurogenic
niches in non-neurogenic regions, generating new neurons for a high period of
time after grafting [57,58]. This opens new perspectives for treating a number
of brain pathologies, including neurodegenerative diseases, but specially for
recovering the lost recent memory and abnormal neurogenesis after hippocampal
injury.
Recently, it has been reported that primitive NSCs (pNSCS) can be easily
obtained from human induced pluripotent stem cells (hPSCS) [59]. These NSCs
express neural stem cells markers (Pax6, Sox2 and Nestin and are negative for
Oct4). They present the advantage that can be expanded for multiple passages,
and can be differentiated into neurons, astrocytes and oligodendrocytes, which
allow them to be useful for treating different neural diseases. Moreover, and
depending on the brain area they can give origin to different neuronal
subtypes, including dopaminergic, GABAergic and motor neurons. This, together
with the fact that only 7 days are needed for inducing hPSCS into pNSCs opens
new therapeutic perspectives which still have to be explored in clinical
trials.
After this brief review about the sources and types of stem cells able to
be infused in human patients, we will focus on four situations that, due to
their high prevalence in the population and their extreme severity, require
rapid action in terms of stem cell treatments.
Cerebral palsy (CP): Cerebral palsy
is a non-progressive disease occurring in 2-2.5/1.000 live births, and is
mainly characterized by motor disorders, although many of the children also
suffer cognitive affectations, speech impairments or absence of language,
hearing and visual affectations (these range from squint to absolute
blindness), seizures, drooling, etc. CP is usually produced by damage to the
developing brain, because of many causes, including maternal infections (such
as those frequently produced by cytomegalovirus) or toxic habits, but also is
produced by asphyxia before birth, hypoxia/ischemia at birth, brain trauma
during labor and delivery or prematurity leading to a brain white matter
affectation known as Periventricular Leukomalacia and parenchymal venous
infarction complicating germinal matrix/intraventricular hemorrhage. Other
causes are related to post-natal infections (i.e., meningitis) or traumas. In
any case, given the age at which it occurs, CP represents a a great public
health problem and tremendous economic costs for the patient's family and the
state. According to a study carried out in Denmark, the cost of CP throughout
the life of one of these patients is around $1.2 million of US dollars for men and
about $1.1 million of US dollars for women [60].
Due to the magnitude of this problem in terms of both personal and familiar
affectations, and social costs, the need exists for urgently finding a
therapeutic solution for children with CP.
Stroke: In Spain, as in
many other developed countries, cerebrovascular diseases are the second main
cause of mortality in the population, and the first in women [61]. In 2011, the
Hospital Morbidity Survey of the National Statistics Institute reported 116.017
strokes and 14.933 transient ischemic episodes; this implies an incidence of
252 strokes and 32 ischemic episodes per 100.000 people. These data presumably
will increase in the coming years due to the habits of life and the aging of
population.
Traumatic brain injury
(TBI): In the case of TBI, the annual incidence of new cases in Spain was
estimated at 200 per 100.000 inhabitants, 40% of them being due to traffic
accidents (data from 2006).
It is clear that, as it occurs in stroke, the high social and sanitary
impact that TBI produces requires urgent measures in terms of prevention but
also of recovery of the neural injuries suffered once the critical episode has
been resolved in the hospital.
TBI is quite different from stroke, since the brain injuries produced after
TBI usually are diffuse and progressive, while in stroke they are generally
restricted to the area affected; the main problem associated with TBI is the
development of a diffuse axonal injury, an event that can lead to instant
death, or progress during days or weeks due to axonal shearing, and subsequent
progressive brain inflammation, leading the patient to a vegetative coma or
permanent disabilities. This is a main reason for treating to develop new
therapeutic strategies in order to prevent these terrific consequences.
Myocardial infarction: This occurs
when the blood supply to a part of the heart decreases or is fully interrupted
because of the blockade of a coronary artery produced by the rupture of an
atherosclerotic plaque. The result is heart damage that can lead to the sudden death
of the patient. Urgent treatmet is therefore needed, with anticoagulants, such
as aspirin, or percutaneous coronary intervention for trying to push open the
affected coronary or perform a thrombolysis. Later, a stent can be placed in
the location in which the coronary artery had been occluded, or a coronary
bypass surgery can be carried out. However, none of these have been reported to
recover the damaged muscle heart, rather they try to prevent the appearance of
a new episode. Hypertension, smoking, obesity, increased LDL/HDL cholesterol
ratio, sedentary life and excessive alcohol intake are risk factorsfor
developing a myocardial infarction along the life.
DISCUSSION
After a brief description of the effects of GH at the neural and
cardiovascular levels, as well as the main types of stem cells used in a series
of treatments, and a schematic description of what four important pathologies
mean, we will now analyze whether it would be feasible to combine the
administration of GH, given its own effects and those exerted by the high
number of trophic factors whose expression is induced by this hormone, with
stem cell treatments in these pathologies, to try to achieve better results.
Cerebral palsy
While many recent studies analyzing the effects of stem cells treatments
describe positive, although rather modest, results in cerebral palsy [63-68],
most of them use cord blood stem cells, a treatment that is impossible to be
carried out in Spain, because national health laws do not allow to store the
umbilical cord of any newborn for its own use. Rather, these umbilical cords
are stored for public use in any patient who could need it in a future; for
example for cancer treatments once the HLA compatibility has been checked. This
does not exclude that if the delivery has taken place in a private hospital,
the family can request the collection of the cord to be stored in a private
stem cells bank located in another country.
Stroke
During the last years many studies analyzed the efficiency of the
application of human adult stem cells from different sources (bone marrow,
umbilical cord, adipose tissue, even menstrual blood, among others) for the
recovery of the disturbed neuronal circuitry and disruption of the blood-brain-barrier
after stroke [72-83]. These are only a small but representative sample of the
high number of published articles about stem cells therapies for the treatment
of stroke. The results have been controversial regarding the efficacy of this type
of therapy. Most likely the finding of significant improvements or, on the
contrary, the lack of beneficial effects depends on multiple factors, such as:
the time elapsed between stroke and treatment, the via of administration of
stem cells (intravenous, intra-arterial, intrathecal) and, perhaps, the age of
the patients at which they are treated, because the reparative properties of
the brain decrease while aging.
Regarding the time elapsed after the stroke occurred and the treatment with
stem cells starts, a clinical trial administering intravenous MSCs in the acute
phase of stroke has being carried out in UK and USA [79], without sigificant
improvements observed at 90 days in neurological outcomes, and another one is
commencing in many european countries including different Spanish hospitals.
However, a recent clinical trial performed in Hong Kong in patients who had
suffered cerebral haemorrhage one year before being treated with intravenous
injections of autologous MSCs showed improvements of motor disabilities and
cognitive impairments over a year after being treated [84].
Due to its nature, TBI is lesser able to be
treated with a local application of stem cells during the acute phase of the
injury [89]. However, a number of preclinical studies indicate that
administration of MSCs seem to be effective for brain repair after TBI in rats
[90-104]. Despite these data, few studies have been done, until now, in human
patients. In fact, when seeking for these studies on the website
Clinicaltrials.gov, only five can be found. Three of them have been completed
(one in children) and two are still recruiting patients (adult TBI Phase 2b).
In 2013, Tian et al. [105] reported the results of
autologous bone marrow MSCs administration by lumbar puncture in 97 patients in
the subacute stage of TBI. Interestingly, 24 of these patients were in
persistent vegetative state and 11 of them showed significant improvements in
consciousness after the treatment, while 27 of 73 patients with severe motor
disorders also showed improvements in motor functions. They concluded that this
kind of therapy is safe and effective, and also, as it seems to be logic, that
young patients improved better than older ones. Another very important and also
logical conclusion was that this therapy has to be applied early in the
subacute stage of TBI for obtaining better results. More recently, an
intravenous autologous bone marrow MSCs was shown to decrease the needs of
intense treatment for decreasing intracranial pressure, the severity of brain
injury and duration of neurointensive cares in children early receiving these
stem cells after TBI [106]. These positive results have been postulated to be
due to the effect of stem cells on the neuroinflammation that TBI produces.
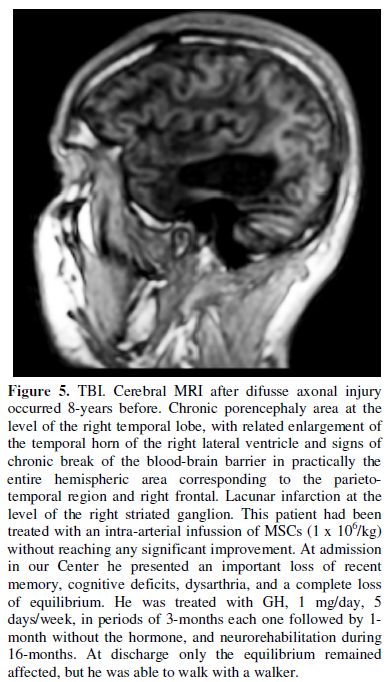
Another recent study in three patients suffering neurological sequelae after
difusse axonal injury, showed that the intrathecal administration of autologous
MSCs resulted in improvements of their neurological situation and a diffuse and
progressive increase in the cerebral metabolism of glucose, as reflected by
positron emission tomography (PET) [107]. Given that glucose is the main
nutrient for neurons, this result clearly indicates that brain activity
improved after the intrathecal administration of MSCs.
But, again, similar results occur when treating
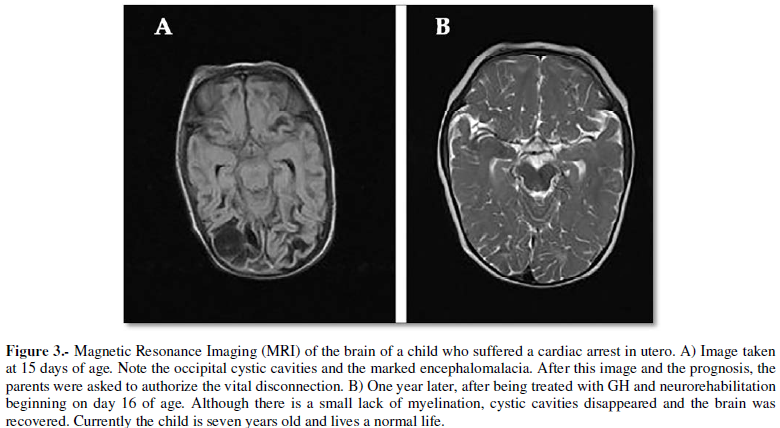
TBI with GH. We were the first to use this hormone (December 2002) at a very
early stage of a TBI that produced diffusse axonal injury, traumatic
subarachnoidal hemorrhage, multiple fronto-temporal and intraventricular
bleeding and brainstem damage; fortunately, the patient had a very good
recovery and eight months after his TBI produced by a car accident, he went
back to his University studies and he reached the degree of European PhD and
lives a fully normal life. We published it 11 years later (25, Case 1). Hence,
GH might also be useful for treating TBI with stem cells transplants, helping
these stem cells to survive, differentiate and release neurotrophic factors.
Moreover, GHD is a common finding in TBI patients.
Myocardial
infarction
However, three years later, the same group
communicated that in response to different forms of stress, these CSCs acquire
a senescent phenotype, thus losing their functional properties as reparative
agents [110]. In order to avoid this problem, these authors proposed the search
of mechanisms able to produce the activation of CSCs in situ and to clarify the mechanisms responsibles for the
senescence to prevent or reverse its presentation [110].
CSCs have been successfully isolated from biopsies
of human myocardium and expanded ex vivo
without any lost of its potential for differentiating into cardiomyocytes and
vascular cells [111], therefore allowing the autologous transplantation back
into the heart mediating, together with the resident CSCs, myocardial
regeneration to a significant degree.
These studies may explain the fact that
intramyocardial injection of MSCs overexpressing the survival factor Akt may
significantly repair infarcted myocardium in rats and improve cardiac function,
as early as 3 days after the injection, despite that only a small number of
MSCs differentiated into cardiomyocytes [112]. It seems to be clear that in
addition of the survival role that Akt plays [113], cytokines and growth
factors released by the impanted MSCs contribute to the results obtained in
that study [112]. In addition, timing of intracoronary transplantation in acute
myocardial infarction is another key factor for positive outcomes, as a recent
meta-analysis of 34 randomized controlled trials shows [114]. Curiously, the
ideal window of time for this therapy ranges from 3 to 7 days, rather than
within 24 h after the acute myocardial infarction, as one would think. Another
key factor for positive outcomes is the number of MSCs administered, no lesser
than 108-109 [115].
Despite these promising previous studies, the
current situation is still far from being clear. A number of preclinical and
clinical studies have analyzed the potential of endothelial progenitor cells
(EPCs) and CSCs for repairing cardiovascular diseases, but while some of these
studies show improvements in left ventricular ejection fraction in patients with
acute myocardial infarction, other results have been poor or no significant
clinical benefit has been observed in many cases [116].
Another source of stem cells, possibly useful for
being used after a cardiac infarction, is the adipose tissue surrounding the
heart. From this tissue MSCs can be isolated, and when injected
intramyocardially in postinfarcted mice and rats enhance myocardial
vascularization, reduce the infarct size and express cardiac and endothelial
markers [117]. However, these stem cells have not yet been tested in clinical
studies [118].
In order to improve the survival and integration
of administered stem cells in the damaged brain or heart, a number of ex vivo modifications of these cells
(including gene therapies for enhancing the therapeutic effects of these cells
or its specific delivery to a particular tissue) or implantable devices
containing them, have been proposed [77,130-138].
As stated above, a number of references indicate
that the administration of growth hormone (GH) might be of great utility when
commencing a therapy with stem cells in any of the pathologies we analyzed.
The rationale of the use of GH together with stem
cells administration comes not only for the already described actions of the
hormone (for instance cells survival, and stem cells proliferation,
differentiation and migration), but also from the fact that GH induces the
expression of a number of factors with known neurotrophic and cardiac activity.
For example, IGF-I, EGF, FGF, VEGF, BDNF, EPO, etc. [1]. In addition, it would
not be necessary a long time GH administration, therefore avoiding the
apparition of possible undesirable side-effects. Even more, GH could be
administered early after the brain or cardiac injuries, before stem cells could
be implanted.
CONCLUSION
Stem cells implants from many different sources are a potential solution
for several acquired neural and cardiac injuries. However, the time window for these
implants achieve maximum restorative effectiveness is still under debate and
dependent on the type and severity of existing damage, as it happens with the
number of cells which need to be administered and the route of administration.
The administration of GH, regardless of whether the patient is GH-deficient or
not, can be an effective tool to make treatments with stem cells more efficient
in these diseases.
ACKNOWLEDGEMENT
This study has been funded by Foundation Foltra (Teo, Spain).
CONFLICT OF INTERESTS
The authors declare that no conflict of interests exists.
1.
Devesa J, Almenglo
C, Devesa P (2016) Multiple effects of growth hormone in the body: Is it really
the hormone for growth? Clin Med Insights: Endocrinol Diabetes 9: 1-25.
2.
Devesa J, Diaz MJ,
Odriozola A, Arce V, Lima L (1991) Neurorregulacion de la expresion de la
secrecion de hormona de crecimiento (GH) y expresion del gen de esta hormona en
pro-y eucariotas. Endocrinologia 38: 33-41.
3.
Devesa J, Devesa P,
Reimunde P (2010) Growth hormone revisited. Med Clin (Barc) 135: 665-670.
4.
Arce VM, Devesa P,
Devesa J (2013) Role of Growth Hormone in the treatment of neural diseases: From
neuroprotection to neural repair. Neurosci Res 76: 179-186.
5.
Caicedo D, Devesa
P, Arce VM, Requena J, Devesa J (2017) Critical lower limb ischemia could
benefit from growth hormone therapy for wound healing and limb salvage. Ther
Adv Cardiovasc Dis.
6.
Pathipati P, Gorba
T, Scheepens A, Goffin V, Sun Y, et al. (2011) Growth hormone and prolactin
regulate human neural stem cell regenerative activity. Neuroscience 190:
409-427.
7.
Devesa P, Agasse F,
Xapelli, Almenglo C, Devesa J, et al. (2014) Growth hormone pathways signaling for cell proliferation and survival
in hippocampal neural precursors from postnatal mice. BMC Neurosci 15: 100.
8.
Scheepens A, Williams CE, Breier
BH, Guan J, Gluckman PD (2000) A role for the somatotropic axis in neural
development, injury and disease. J Pediatr Endocrinol Metab 13: 1483-1491.
9.
Garcia-Aragon
J, Lobie PE, Muscat GE, Gobius KS, Norstedt G, et al. (1992) Prenatal
expression of the growth hormone (GH) receptor/binding protein in the rat: A role
for GH in embryonic and fetal development? Development 114: 869-876.
10.
Turnley AM, Faux CH, Rietze RL,
Coonan JR, Bartlett PF (2002) Suppressor of cytokine signaling 2 regulates
neuronal differentiation by inhibiting growth hormone signaling. Nat Neurosci
5: 1155-1162.
11.
Lobie PE, Garcia-Aragon J, Lincoln DT, Barnard R, Wilcox
JN, et al. (1993) Localization and ontogeny of growth hormone receptor gene
expression in the central nervous system. Brain Res Dev Brain Res 74: 225-233.
12.
Donahue CP, Jensen
RV, Ochiishi T, Eisenstein I, Zhao M, et al. (2002) Transcriptional profiling
reveals regulated genes in the hippocampus during memory formation. Hippocampus
12: 821-833.
13.
Parent JM
(2003) Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist
9: 261-272.
14.
Sun LY, Evans MS, Hsieh J, Panici J, Bartke A
(2005) Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice.
Endocrinology 146: 1138-1144.
15.
Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A
(2005) Local expression of GH and IGF-1 in the hippocampus of GH-deficient
long-lived mice. Neurobiol Aging 26: 929-937.
16.
Scheepens A, Sirimanne ES, Breier
BH, Clark RG, Gluckman PD, et al. (2001) Growth hormone as a neuronal rescue
factor during recovery from CNS injury. Neuroscience 104: 677-687.
17.
Devesa J, Reimunde P, Devesa A,
Souto S, Lopez-Amado M, et al. (2009) Recovery from neurological sequelae
secondary to oncological brain surgery in an adult growth hormone-deficient
patient after growth hormone treatment. J Rehabil Med 41: 775-777.
18.
Maric NP, Doknic M, Pavlovic D,
Pekic S, Stojanovic M, et al. (2010) Psychiatric and neuropsychological changes
in growth hormone-deficient patients after traumatic brain injury in response
to growth hormone therapy. J Endocrinol Invest 33: 770-775.
19.
High WM Jr, Briones-Galang M,
Clark JA, Gilkison C, Mossberg KA, et al. (2010) Effect of growth hormone
replacement therapy on cognition after traumatic brain injury. J Neurotrauma
27: 1565-1575.
20.
Reimunde P,
Rodicio C, Lopez N, Alonso A, Devesa P, et
al. (2010) Effects of recombinant growth hormone replacement and
physical rehabilitation in recovery of gross motor function in children with
cerebral palsy. Ther Clin Risk Manag 6: 585-592.
21.
Reimunde P,
Quintana A, Castanon B, Casteleiro N, Vilarnovo Z, et al. (2011) Effects of
growth hormone (GH) replacement and cognitive rehabilitation in patients with
cognitive disorders after traumatic brain injury. Brain Inj 25: 65-73.
22.
Devesa J, Alonso B, Casteleiro N,
Couto P, Castanon B, et al. (2011) Effects of recombinant growth hormone (GH)
replacement and psychomotor and cognitive stimulation in the neurodevelopment
of GH-deficient (GHD) children with cerebral palsy: A pilot study. Ther Clin
Risk Manag 7: 199-206.
23.
Li RC, Guo SZ,
Raccurt M, Moudilou E, Morel G, et al. (2011) Exogenous growth hormone
attenuates cognitive deficits induced by intermittent hypoxia in rats. Neurosci
196: 237-250.
24.
Song J, Park
K, Lee H, Kim M (2012) The effect of recombinant human growth hormone therapy
in patients with completed stroke: A pilot trial. Ann Rehabil Med 36: 447-457.
25.
Devesa J, Reimunde P, Devesa P,
Barbera M, Arce V (2013) Growth hormone (GH) and brain trauma. Horm Behav 63:
331-344.
26.
Heredia M,
Fuente A, Criado J, Yajeya J, Devesa J,
et al. (2013) Early growth hormone (GH) treatment promotes relevant motor
functional improvement after severe frontal cortex lesion in adult rats. Behav
Brain Res 247: 48-58.
27.
Moreau OK,
Cortet-Rudelli C, Yollin E, Merlen E, Daveluy W, et al. (2013) Growth hormone
replacement therapy in patients with traumatic brain injury. J Neurotrauma 30:
998-1006.
28.
Alba-Betancourt
C, Luna-Acosta JL, Ramirez-Martinez CE, Avila-Gonzalez D, Granados-Avalos E, et
al. (2013) Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury
in embryonic chicken cerebellum. Gen Comp Endocrinol 183: 17-31.
29.
Nyberg F,
Hallberg M (2013) Growth hormone and cognitive function. Nat Rev Endocrinol 9:
357-365.
30.
Rhodin A, von
Ehren M, Skottheim B, Gronbladh A, Ortiz-Nieto F, et al. (2014) Recombinant
human growth hormone improves cognitive capacity in a pain patient exposed to
chronic opioids. Acta Anaesthesiol Scand 58: 759-765.
31.
Zhang H, Han
M, Zhang X, Sun X, Ling F (2014) The effect and mechanism of growth hormone
replacement on cognitive function in rats with traumatic brain injury. PLoS One
9: e108518.
32.
Devesa J, Diaz-Getino G, Rey P,
Garcia-Cancela J, Loures I, et al. (2015) Brain recovery after a plane crash:
Treatment with growth hormone (GH) and neurorehabilitation: A case report. Int
J Mol Sci 16: 30470-30482.
33.
Gardner CJ,
Mattsson AF, Daousi C, Korbonits M, Koltowska-Haggstrom M, et al. (2015) GH deficiency after traumatic brain injury: Improvement in quality
of life with GH therapy: Analysis
of the KIMS database. Eur J Endocrinol 172: 371-381.
34.
Devesa J, Lema H, Zas E, Munin B,
Taboada P, et al. (2016) Learning and memory recoveries in a young girl treated
with growth hormone and neurorehabilitation. J Clin Med 5.
35.
Christophidis LJ, Gorba T,
Gustavsson M, Williams CE, Werther GA, et al. (2009) Growth hormone receptor
immunoreactivity is increased in the subventricular zone of juvenile rat brain
after focal ischemia: A potential role for growth hormone in injury-induced
neurogenesis. Growth Horm IGF Res 19: 497-506.
36.
Devesa P,
Reimunde P, Gallego R, Devesa J, Arce VM (2011) Growth hormone (GH) treatment
may cooperate with locally-produced GH in increasing the proliferative response
of hippocampal progenitors to kainate-induced injury. Brain Inj 25: 503-510.
37.
Almenglo C, Devesa P, Devesa J, Arce VM (2017) GPE
promotes the proliferation and migration of mouse embryonic neural stem cells
and their progeny in vitro. Int J Mol
Sci 18.
38.
Martinez-Moreno
CG, Fleming T, Carranza M, Avila-Mendoza J, Luna M, et al. (2017) Growth hormone protects against kainate
excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal
cells. Exp Eye Res 166: 1-12.
39.
Evans LM,
Davies JS, Anderson RA, Ellis GR, Jackson SK, et al. (2000) The effect of GH
replacement therapy on endothelial function and oxidative stress in adult
growth hormone deficiency. Eur J Endocrinol 142: 254-262.
40.
Boger RH
(1999) Nitric oxide and the mediation of the hemodynamic effects of growth
hormone in humans. J Endocrinol Invest 22: 7581.
41.
Napoli R,
Guardasole V, Angelini V, D'Amico F, Zarra E, et al. (2003) Acute effects of
growth hormone on vascular function in human subjects. J Clin Endocrinol Metab
88: 2817-2820.
42.
Daskalopoulos
EP, Vilaeti AD, Barka E, Mantzouratou P, Kouroupis D, et al. (2015) Attenuation
of post-infarction remodeling in rats by sustained myocardial growth hormone
administration. Growth Factors 12: 1-9.
43.
Kontonika M, Barka
E, Roumpi M, La Rocca V, Lekkas P, et al. (2017) Prolonged intra-myocardial growth
hormone administration ameliorates post-infarction electrophysiologic remodeling
in rats. Growth Factors 35: 1-11.
44.
Takahasi K,
Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and
adult fibroblast cultures by defined factors. Cell 126: 663-676.
45.
Mai Q, Yu Y, Li T, Wang L, Chen MJ, et al. (2007)
Derivation of human embryonic stem cell lines from parthenogenetic blastocysts.
Cell Res 17: 1008-1019.
46.
Civin CI,
Strauss LC, Brovall C, Fackler MJ, Schwartz JF, et al. (1984) Antigenic
analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen
defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133: 157-165.
47.
Tindle RW,
Katz F, Martin H, Watt D, Catovsky D, et al. (1987) BI-3C5 (CD34) defines
multipotential and lineage restricted progenitor cells and their leukaemic
counterparts: Leucocyte typing 111:
White cell differentiation antigens. Oxford University Press.
48.
Nielsen JS,
McNagny KM (2008) Novel functions of the CD34 family. J Cell Sci 121:
3683-3692.
49.
Blanchet MR,
Maltby S, Haddon DJ, Merkens H, Zbytnuik L, et al. (2007) CD34 facilitates the
development of allergic asthma. Blood
110: 2005-2012.
50.
Squillaro T,
Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: An update.
Cell Transplant 25: 829-848.
51.
Vaquero J, Zurita
M, Rico MA, Bonilla C, Aguayo C, et al. (2016) An approach to personalized cell
therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II
clinical trial. Cytotherapy 18: 1025-1036.
52.
Sasaki M, Abe
R, Fujita Y, Ando S, Inokuma D, et al. (2008) Mesenchymal stem cells are
recruited into wounded skin and contribute to wound repair by
transdifferentiation into multiple skin cell type. J Immunol 180: 2581-2587.
53.
Li M, Ikehara S (2013) Bone-marrow-derived mesenchymal stem cells for
organ repair. Stem Cells Int 2013: 132642.
54.
Sanz-Baro R, Garcia-Arranz M, Guadalajara H, de la Quintana P, Herreros
MD, et al. (2015) First-in-human case study: Pregnancy in women with Crohn's
perianal fistula treated with adipose-derived stem cells: A safety study. Stem
Cells Transl Med 4: 598-602.
55.
Garcia-Olmo D, Schwartz DA (2015) Cumulative evidence that mesenchymal
stem cells promote healing of perianal fistulas of patients with Crohn's
disease. Gastroenterology 149: 853-857.
56.
Uchida H, Niizuma
K, Kushida Y, Wakao S, Tominaga T, et al. (2017) Human muse cells reconstruct
neuronal circuitry in subacute lacunar stroke model. Stroke 48: 428-435.
57.
Hattiangady B, Shetty AK (2012) Neural stem cell grafting counteracts
hippocampal injury-mediated impairments in mood, memory and neurogenesis. Stem
Cells Transl Med 1: 696-708.
58.
Shetty AK, Hattiangady B (2016) Grafted subventricular zone neural stem
cells display robust engraftment and similar differentiation properties and
form new neurogenic niches in the young and aged hippocampus. Stem Cells Transl
Med 5: 1204-1215.
59.
Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, et al.
(2013) Efficient and rapid derivation of primitive neural stem cells and
generation of brain subtype neurons from human pluripotent stem cells. Stem
Cells Transl Med 2: 862-870.
60.
Kruse M, Michelsen SI, Flachs EM,
Bronnum-Hansen H, Madsen M, et al. (2009) Lifetime costs of cerebral palsy. Dev
Med Child Neurol 51: 622-628.
61.
Brea A,
Laclaustra M, Martorell E, Pedragosa A (2013) Epidemiology of cerebrovascular
disease in Spain. Clin Invest Arterioscl 25: 211-217.
62.
Koh SH, Park
HH (2017) Neurogenesis in stroke recovery. Transl Stroke Res 8: 3-13.
63.
Abi Chahine
NH, Wehbe TW, Hilal RA, Zoghbi VV, Melki AE, et al. (2016) Treatment of cerebral
palsy with stem cells: A report of 17 cases. Int J Stem Cells 9: 90-95.
64.
Park KI, Lee YH, Rah WJ, Jo SH, Park SB, et al. (2017) Effect of
intravenous infusion of G-CSF-mobilized peripheral blood mononuclear cells on
upper extremity function in cerebral palsy children. Ann Rehab Med 41: 113-120.
65.
Liu X, Fu X, Dai G, Wang X, Zhang Z, et al. (2017) Comparative analysis
of curative effect of bone marrow mesenchymal stem cell and bone marrow
mononuclear cell transplantation for spastic cerebral palsy. J Transl Med 15:
48.
66.
Nguyen LT, Nguyen AT, Vu CD, Ngo DV, Bui AV (2017) Outcomes of autologous
bone marrow mononuclear cells for cerebral palsy: An open label uncontrolled
clinical trial. BMC Pediatr 17: 104.
67.
McDonald CA, Fahey MC, Jenkin G, Miller SL (2017) Umbilical cord blood
cells for treatment of cerebral palsy; timing and treatment options. Pediatr
Res Sep 22.
68.
Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, et al. (2017) Effect
of autologous cord blood infusion on motor function and brain connectivity in
young children with cerebral palsy: a randomized, placebo-controlled trial.
Stem Cells Transl Med 6: 2071-2078.
69.
Kulak-Bejda A,
Kulak P, Bejda G, Krajewska-Kulak E, et al. (2016) Stem cells therapy in
cerebral palsy: A systematic review. Brain Dev 38: 699-705.
70.
Devesa J,
Devesa P, Reimunde P, Arce V (2012) Growth hormone and kynesitherapy for bain
injury recovery. In: Brain Injury. Pathogenesis, monitoring, recovery and
management. InTech, Rijeka, Croatia.
71.
Devesa J,
Casteleiro N, Rodicio C, López N, Reimunde P (2010) Growth hormone deficiency
and cerebral palsy. Ther Clin Risk Manag 6: 413-418.
72.
Barcena-Orbe A, Rodríguez-Arias CA, Rivero-Martin B, Canizal-Garcia JM, Mestre-Moreiro
C, et al. (2006) Overview of head injury. Neurocirugia (Astur) 17: 495-518.
73.
Ding DC, Shyu WC, Lin SZ, Li H (2006) Current concepts in adult stem cell
therapy for stroke. Curr Med Chem 13: 3565-3574.
74.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, et al. (2014)
Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke:
A multicentric, randomized trial. Stroke 45: 3618-3624.
75.
Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-León M, et
al. (2015) Intra-arterial bone marrow mononuclear cells (BM-MNCs)
transplantation in acute ischemic stroke (IBIS trial): Protocol of a phase II,
randomized, dose-finding, controlled multicenter trial. Int J Stroke 10: 1149-1152.
76.
Azad TD, Veeravagu A, Steinberg GK (2016) Neurorestoration after stroke.
Neurosurg Focus 40: E2.
77.
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD,
Coburn ML, et al. (2016) Clinical outcomes of transplanted modified bone
marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 47:
1817-1824.
78.
Kumar A, Prasad
M, Jali VP, Pandit AK, Misra S, et al. (2017) Bone marrow mononuclear cell
therapy in ischaemic stroke: A systematic review. Acta Neurol Scand 135:
496-506.
79.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, et al. (2017) Safety
and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS):
A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol
16: 360-368.
80.
Kenmuir CL,
Wechsler LR (2017) Update on cell therapy for stroke. Stroke Vasc Neurol 2:
59-64.
81.
Detante O,
Moisan A, Hommel M, Jaillard A (2017) Controlled clinical trials of cell
therapy in stroke: Meta-analysis at six months after treatment. Int J Stroke
12: 748-751.
82.
Huang H, Lin F, Jiang J, Chen Y, Mei A, et al. (2017) Effects of
intra-arterial transplantation of adipose-derived stem cells on the expression
of netrin-1 and its receptor DCC in the peri-infarct cortex after experimental stroke.
Stem Cell Res Ther 8: 223.
83.
Reis C, Wilkinson
M, Reis H, Akyol O, Gospodarev V, et al. (2017) A look into stem cell therapy:
Exploring the options for treatment of ischemic stroke. Stem Cells Int.
84.
Tsang KS, Ng
CPS, Zhu XL, Wong GKC, Lu G, et al. (2017) Phase I/II randomized controlled
trial of autologous bone marrow-derived mesenchymal stem cell therapy for
chronic stroke. World J Stem Cells 9: 133-143.
85.
Duan X, Lu L,
Wang Y, Zhang F, Mao J, et al. (2017) The long-term fate of mesenchymal stem
cells labeled with magnetic resonance imaging-visible polymersomes in cerebral
ischemia. Int J Nanomed 12: 6705-6719.
86.
Seo HG, Yi Y, Oh BM, Paik NJ (2017) Neuroprotective effect
of secreted factors from human adipose stem
cells in a rat stroke model. Neurol Res 39: 1114-1124.
87.
Wei L, Wei ZZ,
Jiang MQ, Mohamad O, Yu SP (2017) Stem cell transplantation therapy for
multifaceted therapeutic benefits after stroke. Prog Neurobiol 157: 49-78.
88.
Chen L, Qiu R,
Li L, He D, Lv H, et al. (2014) The role of exogenous neural stem cells
transplantation in cerebral ischemic stroke. J Biomed Nanotechnol 10:
3219-3230.
89.
Cox CS (2017) Cellular therapy for traumatic neurological injury. Pediatr
Res.
90.
Longhi L, Zanier ER, Royo N, Stochetti N, McIntosh TK (2005) Stem cell
transplantation as a therapeutic strategy for traumatic brain injury. Transpl
Immunol 15: 143-148.
91.
Chen Q, Long Y, Yuan X, Zou L, Sun J, et al. (2005) Protective effects of
bone marrow stromal cell transplantation in injured rodent brain: Synthesis of
neurotrophic factors. J Neurosci Res 80: 611-619.
92.
Xue S, Zhang HT, Zhang P, Luo J, Chen ZZ, et al. (2010) Functional
endothelial progenitor cells derived from adipose tissue show beneficial effect
on cell therapy of traumatic brain injury. Neurosci Lett 473: 186-191.
93.
Walker PA, Harting MT, Jimenez F, Shah SK, Pati S, et al. (2010) Direct
intrathecal implantation of mesenchymal stromal cells leads to enhanced
neuroprotection via an NFkappaB-mediated increase in interleukin-6 production.
Stem Cells Dev 19: 867-876.
94.
Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, et al. (2011)
Mesenchymal stem cell therapy modulates the inflammatory response in
experimental traumatic brain injury. Neurol Res Int 2011: 564089.
95.
Grigorian AS, Gilerovich EG, Pavlichenko NN, Kruglyakov PV, Sokolova IB,
et al. (2011) Effect of transplantation of mesenchymal stem cells on neuronal
survival and formation of a glial scar in the brain of rats with severe traumatic
brain injury. Bull Exp Biol Med 150: 551-555.
96.
Lundberg J, Sodersten E, Sundstrom E, Le Blanc K, Andersson T, et al.
(2012) Targeted intra-arterial transplantation of stem cells to the injured CNS
is more effective than intravenous administration: Engraftment is dependent on
cell type and adhesion molecule expression. Cell Transplant 21: 333-343.
97.
Jiang J, Bu X, Liu M, Cheng P (2012) Transplantation of autologous bone
marrow-derived mesenchymal stem cells for traumatic brain injury. Neural
Regen Res 5: 46-53.
98.
Zhang R, Liu Y, Yan K, Chen L, Chen XR, et al. (2013) Anti-inflammatory
and immunomodulatory mechanisms of mesenchymal stem cell transplantation in
experimental traumatic brain injury. J Neuroinflammation 10: 106.
99.
Anbari F, Khalili MA, Bahrami AR, Khoradmehr A, Sadeghian F, et al.
(2014) Intravenous transplantation of bone marrow mesenchymal stem cells promotes
neural regeneration after traumatic brain injury. Neural Regen Res 9: 919-923.
100.Peng W, Sun J, Sheng C, Wang
Z, Wang Y, et al. (2015) Systematic review and meta-analysis of efficacy
of mesenchymal stem cells on locomotor recovery in animal models of traumatic
brain injury. Stem Cell Res Ther 6: 47.
101.Turtzo LC, Budde MD, Dean
DD, Gold EM, Lewis BK, et al. (2015) Failure of intravenous or intracardiac
delivery of mesenchymal stromal cells to improve outcomes after focal traumatic
brain injury in the female rat. PLoS One 10: e0126551.
102.Mishra SK, Rana P, Khushu
S, Gangenahalli G (2017) Therapeutic prospective of infused allogenic cultured mesenchymal
stem cells in traumatic brain injury mice: A longitudinal proton magnetic
resonance spectroscopy assessment. Stem Cells Transl Med 6: 316-329.
103.Dori I, Petrakis S, Giannakopoulou
A, Bekiari C, Grivas I, et al. (2017) Seven days post-injury fate and effects
of genetically labelled adipose-derived mesenchymal cells on a rat traumatic
brain injury experimental model. Histol Histopathol 32: 1041-1055.
104.Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, et al. (2017)
Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol
8: 28.
105.Tian C, Wang X, Wang X, Wang L, Wang X, et al. (2013) Autologous bone
marrow mesenchymal stem cell
therapy in the subacute stage of traumatic
brain injury by lumbar puncture.
Exp Clin Transplant 11: 176-181.
106.Liao GP, Harting MT, Hetz RA,
Walker PA, Shah SK, et al. (2015) Autologous bone marrow mononuclear cells reduce
therapeutic intensity for severe traumatic brain injury in children. Pediatr
Crit Care Med 16: 245-255.
107.Vaquero J, Zurita M, Bonilla
C, Fernández C, Rubio JJ, et al. (2017) Progressive increase in brain glucose
metabolism after intrathecal administration of autologous mesenchymal stromal
cells in patients with diffuse axonal injury. Cytotherapy 19: 88-94.
108.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, et al. (1999) Autologous
transplantation of bone marrow cells improves damaged heart function.
Circulation 100: II247-256.
109.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, et al. (2003)
Adult cardiac stem cells are multipotent and support myocardial regeneration.
Cell 114: 763-776.
110.Torella D, Ellison GM, Méndez-Ferrer
S, Ibañez B, Nadal-Ginard B (2006) Resident human cardiac stem cells: Role in
cardiac cellular homeostasis and potential for myocardial regeneration. Nat
Clin Pract Cardiovasc Med Suppl 1: S8-13.
111.Barile L, Messina E,
Giacomello A, Marbán E (2007) Endogenous cardiac stem cells. Prog Cardiovasc
Dis 50: 31-48.
112.Noiseux N, Gnecchi M, Lopez-Ilasaca
M, Zhang L, Solomon SD, et al. (2006) Mesenchymal stem cells overexpressing Akt
dramatically repair infarcted myocardium and improve cardiac function despite
infrequent cellular fusion or differentiation. Mol Ther 14: 640-650.
113.Costoya JA, Finidori J, Moutoussamy
S, Senarís R, Devesa J, et al. (1999) Activation of growth hormone receptor
delivers an antiapoptotic signal: Evidence for a role of Akt in this pathway.
Endocrinology 140: 5937-5943.
114.Xu JY, Liu D, Zhong Y, Huang RC (2017) Effects of timing on
intracoronary autologous bone marrow-derived cell transplantation in acute
myocardial infarction: A meta-analysis of randomized controlled trials. Stem
Cell Res Ther 8: 231.
115.Xu JY, Cai WY, Tian M, Liu D, Huang RC (2016) Stem cell
transplantation dose in patients with acute myocardial infarction: A
meta-analysis. Chronic Dis Transl Med 2: 92-101.
116.Bianconi V, Sahebkar A, Kovanen P, Bagaglia F, Ricciuti B, et al.
(2017) Endothelial and cardiac progenitor cells for cardiovascular repair: A
controversial paradigm in cell therapy. Pharmacol Ther pii:
S0163-7258(17)30214-0.
117.Bayes-Genis A, Soler-Botija C, Farré J, Sepúlveda P, Raya A, et al.
(2010) Human progenitor cells derived from cardiac adipose tissue ameliorate
myocardial infarction in rodents. J Mol Cell Cardio l49: 771-780.
118.Roura S, Gálvez-Montón C, Mirabel C, Vives J, et al. (2017)
Mesenchymal stem cells for cardiac repair: Are the actors ready for the
clinical scenario? Stem Cell Res Ther 8: 238.
119.Sacca L, Cittadini A, Fazio S (1994) Growth hormone and the heart. Endocr Rev 15: 555-573.
120.Li Q, Li B, Wang X, Leri A, Jana KP, et al. (1997) Overexpression of
insulin-like growth factor-I in mice protects from myocyte death after
infarction, attenuating ventricular dilation, wall stress and cardiac
hypertrophy. J Clin Invest 100:
1991-1999.
121.Timsit J, Riou B, Bertherat J, Wisnewsky C, Kato NS, et al. (1990)
Effects of chronic growth hormone hypersecretion on intrinsic contractility,
energetics, isomyosin pattern and myosin adenosinetriphosphatase activity of
ratleft ventricle. J Clin Invest
86: 507-515.
122.Cittadini A, Ishiguro Y, Stromer H, Spindler M, Moses AC, et al.
(1998) Insulin-like growth factor-1 but not growth hormone augments mammalian
myocardial contractility by sensitizing the myofilament to Ca2+
through a wortmannin-sensitive pathway: Studies in rat and ferret isolated
muscles. Circ Res 83: 50-59.
123.Mayoux E, Ventura-Clapier R, Timsit J, Behar-Cohen F, Hoffmann C, et
al. (1993) Mechanical properties of rat cardiac skinned fibers are altered by
chronic growth hormone hypersecretion. Circ
Res 72: 57-64.
124.Bruel A, Oxlund H (1995) Biosynthetic growth hormone increase the
collagen deposition rate in rat aorta and heart. Eur J Endocrinol 132: 195-199.
125.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, et al. (1995)
Cardioprotective effect of insulin-like growth factor I in myocardial ischemia
followed by reperfusion. Proc Natl
Acad Sci U S A 92: 8031-8035.
126.Ren J (2002) Short-term administration of insulin-like growth factor
(IGF-1) does not induce myocardial IGF-1 resistance. Growth Horm IGF Res 12: 162-168.
127.Kusano K, Tsutsumi Y, Dean J, Gavin M, Ma H, et al. (2007) Long-term
stable expression of human growth hormone by rAAV promotes myocardial
protection post-myocardial infarction. J
Mol Cell Cardiol 42: 390-399.
128.Rong SL, Lu YX, Liao YH, Wang XL, Wang YJ, et al. (2008) Effects of
transplanted myoblasts transfected with human growth hormone gene on
improvement of ventricular function of rats. Chin Med J 121: 347-354.
129.Nakajima K, Fujita J, Matsui M, Tohyama S, Tamura N, et al. (2015)
Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and
angiogenesis to ameliorate cardiac function after myocardial infarction. PLos
One 10: e0133308.
130.Helie A, Brinker T (2011) Clinical translation of stem cell therapy in
traumatic brain inury: The potential of encapsulated mesenchymal cell
biodelivery of glucagon-like peptide-1. Dialogues Clin Neurosci 13: 279-286.
131.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, et al. (2016)
Intravenous administration of xenogenic adipose-derived mesenchymal stem cells
(ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and
preserved neurological function in rat after acute ischemic stroke. Oncotarget
7: 74537-74556.
132.Bang OY, Kim BH, Cha JM, Moon GJ (2016) Adult stem cell therapy for
stroke: Challenges and progress. J Stroke 18: 256-266.
133.Chung CY, Lin MH, Lee IN, Lee TH, Lee MH, et al. (2017) Brain-derived
neurotrophic factor loaded PS80 PBCA nanocarrier for in vitro neural differentiation of mouse induced pluripotent stem cells.
Int J Mol Sci 18: E663.
134.Wang TW, Chang KC, Chen LH, Liao SY, Yeh CW, et al. (2017) Effects of
an injectable functionalized self-assembling nanopeptide hydrogel on
angiogenesis and neurogenesis for regeneration of the central nervous system.
Nanoscale 9: 16281-16292.
135.Shichinohe H, Kawabori
M, Iijima H, Teramoto T, Abumiya T, et al. (2017) Research on advanced intervention using novel bone marrow stem cell
(RAINBOW): A study protocol for a phase I, open-label, uncontrolled,
dose-response trial of autologous bone marrow stromal cell transplantation in
patients with acute ischemic stroke. BMC Neurol 17: 179.
136.Tseng KY, Anttila JE, Khodosevich K, Tuominen RK, Lindahl M, et al.
(2017) MANF promotes differentiation and migration of neural progenitor cells with
potential neural regenerative effects in stroke. Mol Ther.
137.Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2016) Stem cell
therapy for chronic ischaemic heart disease and congestive heart failure.
Cochrane Database Syst Rev 2: CD007888.
138.Lewis FC, Kumar SD, Ellison-Hughes GM (2017) Non-invasive strategies
for stimulating endogenous repair and regenerative mechanisms in the damaged
heart. Pharmacol Res.
2.
Devesa J, Diaz MJ,
Odriozola A, Arce V, Lima L (1991) Neurorregulacion de la expresion de la
secrecion de hormona de crecimiento (GH) y expresion del gen de esta hormona en
pro-y eucariotas. Endocrinologia 38: 33-41.
3.
Devesa J, Devesa P,
Reimunde P (2010) Growth hormone revisited. Med Clin (Barc) 135: 665-670.
4.
Arce VM, Devesa P,
Devesa J (2013) Role of Growth Hormone in the treatment of neural diseases: From
neuroprotection to neural repair. Neurosci Res 76: 179-186.
5.
Caicedo D, Devesa
P, Arce VM, Requena J, Devesa J (2017) Critical lower limb ischemia could
benefit from growth hormone therapy for wound healing and limb salvage. Ther
Adv Cardiovasc Dis.
6.
Pathipati P, Gorba
T, Scheepens A, Goffin V, Sun Y, et al. (2011) Growth hormone and prolactin
regulate human neural stem cell regenerative activity. Neuroscience 190:
409-427.
7.
Devesa P, Agasse F,
Xapelli, Almenglo C, Devesa J, et al. (2014) Growth hormone pathways signaling for cell proliferation and survival
in hippocampal neural precursors from postnatal mice. BMC Neurosci 15: 100.
8.
Scheepens A, Williams CE, Breier
BH, Guan J, Gluckman PD (2000) A role for the somatotropic axis in neural
development, injury and disease. J Pediatr Endocrinol Metab 13: 1483-1491.
9.
Garcia-Aragon
J, Lobie PE, Muscat GE, Gobius KS, Norstedt G, et al. (1992) Prenatal
expression of the growth hormone (GH) receptor/binding protein in the rat: A role
for GH in embryonic and fetal development? Development 114: 869-876.
10.
Turnley AM, Faux CH, Rietze RL,
Coonan JR, Bartlett PF (2002) Suppressor of cytokine signaling 2 regulates
neuronal differentiation by inhibiting growth hormone signaling. Nat Neurosci
5: 1155-1162.
11.
Lobie PE, Garcia-Aragon J, Lincoln DT, Barnard R, Wilcox
JN, et al. (1993) Localization and ontogeny of growth hormone receptor gene
expression in the central nervous system. Brain Res Dev Brain Res 74: 225-233.
12.
Donahue CP, Jensen
RV, Ochiishi T, Eisenstein I, Zhao M, et al. (2002) Transcriptional profiling
reveals regulated genes in the hippocampus during memory formation. Hippocampus
12: 821-833.
13.
Parent JM
(2003) Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist
9: 261-272.
14.
Sun LY, Evans MS, Hsieh J, Panici J, Bartke A
(2005) Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice.
Endocrinology 146: 1138-1144.
15.
Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A
(2005) Local expression of GH and IGF-1 in the hippocampus of GH-deficient
long-lived mice. Neurobiol Aging 26: 929-937.
16.
Scheepens A, Sirimanne ES, Breier
BH, Clark RG, Gluckman PD, et al. (2001) Growth hormone as a neuronal rescue
factor during recovery from CNS injury. Neuroscience 104: 677-687.
17.
Devesa J, Reimunde P, Devesa A,
Souto S, Lopez-Amado M, et al. (2009) Recovery from neurological sequelae
secondary to oncological brain surgery in an adult growth hormone-deficient
patient after growth hormone treatment. J Rehabil Med 41: 775-777.
18.
Maric NP, Doknic M, Pavlovic D,
Pekic S, Stojanovic M, et al. (2010) Psychiatric and neuropsychological changes
in growth hormone-deficient patients after traumatic brain injury in response
to growth hormone therapy. J Endocrinol Invest 33: 770-775.
19.
High WM Jr, Briones-Galang M,
Clark JA, Gilkison C, Mossberg KA, et al. (2010) Effect of growth hormone
replacement therapy on cognition after traumatic brain injury. J Neurotrauma
27: 1565-1575.
20.
Reimunde P,
Rodicio C, Lopez N, Alonso A, Devesa P, et
al. (2010) Effects of recombinant growth hormone replacement and
physical rehabilitation in recovery of gross motor function in children with
cerebral palsy. Ther Clin Risk Manag 6: 585-592.
21.
Reimunde P,
Quintana A, Castanon B, Casteleiro N, Vilarnovo Z, et al. (2011) Effects of
growth hormone (GH) replacement and cognitive rehabilitation in patients with
cognitive disorders after traumatic brain injury. Brain Inj 25: 65-73.
22.
Devesa J, Alonso B, Casteleiro N,
Couto P, Castanon B, et al. (2011) Effects of recombinant growth hormone (GH)
replacement and psychomotor and cognitive stimulation in the neurodevelopment
of GH-deficient (GHD) children with cerebral palsy: A pilot study. Ther Clin
Risk Manag 7: 199-206.
23.
Li RC, Guo SZ,
Raccurt M, Moudilou E, Morel G, et al. (2011) Exogenous growth hormone
attenuates cognitive deficits induced by intermittent hypoxia in rats. Neurosci
196: 237-250.
24.
Song J, Park
K, Lee H, Kim M (2012) The effect of recombinant human growth hormone therapy
in patients with completed stroke: A pilot trial. Ann Rehabil Med 36: 447-457.
25.
Devesa J, Reimunde P, Devesa P,
Barbera M, Arce V (2013) Growth hormone (GH) and brain trauma. Horm Behav 63:
331-344.
26.
Heredia M,
Fuente A, Criado J, Yajeya J, Devesa J,
et al. (2013) Early growth hormone (GH) treatment promotes relevant motor
functional improvement after severe frontal cortex lesion in adult rats. Behav
Brain Res 247: 48-58.
27.
Moreau OK,
Cortet-Rudelli C, Yollin E, Merlen E, Daveluy W, et al. (2013) Growth hormone
replacement therapy in patients with traumatic brain injury. J Neurotrauma 30:
998-1006.
28.
Alba-Betancourt
C, Luna-Acosta JL, Ramirez-Martinez CE, Avila-Gonzalez D, Granados-Avalos E, et
al. (2013) Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury
in embryonic chicken cerebellum. Gen Comp Endocrinol 183: 17-31.
29.
Nyberg F,
Hallberg M (2013) Growth hormone and cognitive function. Nat Rev Endocrinol 9:
357-365.
30.
Rhodin A, von
Ehren M, Skottheim B, Gronbladh A, Ortiz-Nieto F, et al. (2014) Recombinant
human growth hormone improves cognitive capacity in a pain patient exposed to
chronic opioids. Acta Anaesthesiol Scand 58: 759-765.
31.
Zhang H, Han
M, Zhang X, Sun X, Ling F (2014) The effect and mechanism of growth hormone
replacement on cognitive function in rats with traumatic brain injury. PLoS One
9: e108518.
32.
Devesa J, Diaz-Getino G, Rey P,
Garcia-Cancela J, Loures I, et al. (2015) Brain recovery after a plane crash:
Treatment with growth hormone (GH) and neurorehabilitation: A case report. Int
J Mol Sci 16: 30470-30482.
33.
Gardner CJ,
Mattsson AF, Daousi C, Korbonits M, Koltowska-Haggstrom M, et al. (2015) GH deficiency after traumatic brain injury: Improvement in quality
of life with GH therapy: Analysis
of the KIMS database. Eur J Endocrinol 172: 371-381.
34.
Devesa J, Lema H, Zas E, Munin B,
Taboada P, et al. (2016) Learning and memory recoveries in a young girl treated
with growth hormone and neurorehabilitation. J Clin Med 5.
35.
Christophidis LJ, Gorba T,
Gustavsson M, Williams CE, Werther GA, et al. (2009) Growth hormone receptor
immunoreactivity is increased in the subventricular zone of juvenile rat brain
after focal ischemia: A potential role for growth hormone in injury-induced
neurogenesis. Growth Horm IGF Res 19: 497-506.
36.
Devesa P,
Reimunde P, Gallego R, Devesa J, Arce VM (2011) Growth hormone (GH) treatment
may cooperate with locally-produced GH in increasing the proliferative response
of hippocampal progenitors to kainate-induced injury. Brain Inj 25: 503-510.
37.
Almenglo C, Devesa P, Devesa J, Arce VM (2017) GPE
promotes the proliferation and migration of mouse embryonic neural stem cells
and their progeny in vitro. Int J Mol
Sci 18.
38.
Martinez-Moreno
CG, Fleming T, Carranza M, Avila-Mendoza J, Luna M, et al. (2017) Growth hormone protects against kainate
excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal
cells. Exp Eye Res 166: 1-12.
39.
Evans LM,
Davies JS, Anderson RA, Ellis GR, Jackson SK, et al. (2000) The effect of GH
replacement therapy on endothelial function and oxidative stress in adult
growth hormone deficiency. Eur J Endocrinol 142: 254-262.
40.
Boger RH
(1999) Nitric oxide and the mediation of the hemodynamic effects of growth
hormone in humans. J Endocrinol Invest 22: 7581.
41.
Napoli R,
Guardasole V, Angelini V, D'Amico F, Zarra E, et al. (2003) Acute effects of
growth hormone on vascular function in human subjects. J Clin Endocrinol Metab
88: 2817-2820.
42.
Daskalopoulos
EP, Vilaeti AD, Barka E, Mantzouratou P, Kouroupis D, et al. (2015) Attenuation
of post-infarction remodeling in rats by sustained myocardial growth hormone
administration. Growth Factors 12: 1-9.
43.
Kontonika M, Barka
E, Roumpi M, La Rocca V, Lekkas P, et al. (2017) Prolonged intra-myocardial growth
hormone administration ameliorates post-infarction electrophysiologic remodeling
in rats. Growth Factors 35: 1-11.
44.
Takahasi K,
Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and
adult fibroblast cultures by defined factors. Cell 126: 663-676.
45.
Mai Q, Yu Y, Li T, Wang L, Chen MJ, et al. (2007)
Derivation of human embryonic stem cell lines from parthenogenetic blastocysts.
Cell Res 17: 1008-1019.
46.
Civin CI,
Strauss LC, Brovall C, Fackler MJ, Schwartz JF, et al. (1984) Antigenic
analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen
defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133: 157-165.
47.
Tindle RW,
Katz F, Martin H, Watt D, Catovsky D, et al. (1987) BI-3C5 (CD34) defines
multipotential and lineage restricted progenitor cells and their leukaemic
counterparts: Leucocyte typing 111:
White cell differentiation antigens. Oxford University Press.
48.
Nielsen JS,
McNagny KM (2008) Novel functions of the CD34 family. J Cell Sci 121:
3683-3692.
49.
Blanchet MR,
Maltby S, Haddon DJ, Merkens H, Zbytnuik L, et al. (2007) CD34 facilitates the
development of allergic asthma. Blood
110: 2005-2012.
50.
Squillaro T,
Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: An update.
Cell Transplant 25: 829-848.
51.
Vaquero J, Zurita
M, Rico MA, Bonilla C, Aguayo C, et al. (2016) An approach to personalized cell
therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II
clinical trial. Cytotherapy 18: 1025-1036.
52.
Sasaki M, Abe
R, Fujita Y, Ando S, Inokuma D, et al. (2008) Mesenchymal stem cells are
recruited into wounded skin and contribute to wound repair by
transdifferentiation into multiple skin cell type. J Immunol 180: 2581-2587.
53.
Li M, Ikehara S (2013) Bone-marrow-derived mesenchymal stem cells for
organ repair. Stem Cells Int 2013: 132642.
54.
Sanz-Baro R, Garcia-Arranz M, Guadalajara H, de la Quintana P, Herreros
MD, et al. (2015) First-in-human case study: Pregnancy in women with Crohn's
perianal fistula treated with adipose-derived stem cells: A safety study. Stem
Cells Transl Med 4: 598-602.
55.
Garcia-Olmo D, Schwartz DA (2015) Cumulative evidence that mesenchymal
stem cells promote healing of perianal fistulas of patients with Crohn's
disease. Gastroenterology 149: 853-857.
56.
Uchida H, Niizuma
K, Kushida Y, Wakao S, Tominaga T, et al. (2017) Human muse cells reconstruct
neuronal circuitry in subacute lacunar stroke model. Stroke 48: 428-435.
57.
Hattiangady B, Shetty AK (2012) Neural stem cell grafting counteracts
hippocampal injury-mediated impairments in mood, memory and neurogenesis. Stem
Cells Transl Med 1: 696-708.
58.
Shetty AK, Hattiangady B (2016) Grafted subventricular zone neural stem
cells display robust engraftment and similar differentiation properties and
form new neurogenic niches in the young and aged hippocampus. Stem Cells Transl
Med 5: 1204-1215.
59.
Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, et al.
(2013) Efficient and rapid derivation of primitive neural stem cells and
generation of brain subtype neurons from human pluripotent stem cells. Stem
Cells Transl Med 2: 862-870.
60.
Kruse M, Michelsen SI, Flachs EM,
Bronnum-Hansen H, Madsen M, et al. (2009) Lifetime costs of cerebral palsy. Dev
Med Child Neurol 51: 622-628.
61.
Brea A,
Laclaustra M, Martorell E, Pedragosa A (2013) Epidemiology of cerebrovascular
disease in Spain. Clin Invest Arterioscl 25: 211-217.
62.
Koh SH, Park
HH (2017) Neurogenesis in stroke recovery. Transl Stroke Res 8: 3-13.
63.
Abi Chahine
NH, Wehbe TW, Hilal RA, Zoghbi VV, Melki AE, et al. (2016) Treatment of cerebral
palsy with stem cells: A report of 17 cases. Int J Stem Cells 9: 90-95.
64.
Park KI, Lee YH, Rah WJ, Jo SH, Park SB, et al. (2017) Effect of
intravenous infusion of G-CSF-mobilized peripheral blood mononuclear cells on
upper extremity function in cerebral palsy children. Ann Rehab Med 41: 113-120.
65.
Liu X, Fu X, Dai G, Wang X, Zhang Z, et al. (2017) Comparative analysis
of curative effect of bone marrow mesenchymal stem cell and bone marrow
mononuclear cell transplantation for spastic cerebral palsy. J Transl Med 15:
48.
66.
Nguyen LT, Nguyen AT, Vu CD, Ngo DV, Bui AV (2017) Outcomes of autologous
bone marrow mononuclear cells for cerebral palsy: An open label uncontrolled
clinical trial. BMC Pediatr 17: 104.
67.
McDonald CA, Fahey MC, Jenkin G, Miller SL (2017) Umbilical cord blood
cells for treatment of cerebral palsy; timing and treatment options. Pediatr
Res Sep 22.
68.
Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, et al. (2017) Effect
of autologous cord blood infusion on motor function and brain connectivity in
young children with cerebral palsy: a randomized, placebo-controlled trial.
Stem Cells Transl Med 6: 2071-2078.
69.
Kulak-Bejda A,
Kulak P, Bejda G, Krajewska-Kulak E, et al. (2016) Stem cells therapy in
cerebral palsy: A systematic review. Brain Dev 38: 699-705.
70.
Devesa J,
Devesa P, Reimunde P, Arce V (2012) Growth hormone and kynesitherapy for bain
injury recovery. In: Brain Injury. Pathogenesis, monitoring, recovery and
management. InTech, Rijeka, Croatia.
71.
Devesa J,
Casteleiro N, Rodicio C, López N, Reimunde P (2010) Growth hormone deficiency
and cerebral palsy. Ther Clin Risk Manag 6: 413-418.
72.
Barcena-Orbe A, Rodríguez-Arias CA, Rivero-Martin B, Canizal-Garcia JM, Mestre-Moreiro
C, et al. (2006) Overview of head injury. Neurocirugia (Astur) 17: 495-518.
73.
Ding DC, Shyu WC, Lin SZ, Li H (2006) Current concepts in adult stem cell
therapy for stroke. Curr Med Chem 13: 3565-3574.
74.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, et al. (2014)
Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke:
A multicentric, randomized trial. Stroke 45: 3618-3624.
75.
Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-León M, et
al. (2015) Intra-arterial bone marrow mononuclear cells (BM-MNCs)
transplantation in acute ischemic stroke (IBIS trial): Protocol of a phase II,
randomized, dose-finding, controlled multicenter trial. Int J Stroke 10: 1149-1152.
76.
Azad TD, Veeravagu A, Steinberg GK (2016) Neurorestoration after stroke.
Neurosurg Focus 40: E2.
77.
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD,
Coburn ML, et al. (2016) Clinical outcomes of transplanted modified bone
marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 47:
1817-1824.
78.
Kumar A, Prasad
M, Jali VP, Pandit AK, Misra S, et al. (2017) Bone marrow mononuclear cell
therapy in ischaemic stroke: A systematic review. Acta Neurol Scand 135:
496-506.
79.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, et al. (2017) Safety
and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS):
A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol
16: 360-368.
80.
Kenmuir CL,
Wechsler LR (2017) Update on cell therapy for stroke. Stroke Vasc Neurol 2:
59-64.
81.
Detante O,
Moisan A, Hommel M, Jaillard A (2017) Controlled clinical trials of cell
therapy in stroke: Meta-analysis at six months after treatment. Int J Stroke
12: 748-751.
82.
Huang H, Lin F, Jiang J, Chen Y, Mei A, et al. (2017) Effects of
intra-arterial transplantation of adipose-derived stem cells on the expression
of netrin-1 and its receptor DCC in the peri-infarct cortex after experimental stroke.
Stem Cell Res Ther 8: 223.
83.
Reis C, Wilkinson
M, Reis H, Akyol O, Gospodarev V, et al. (2017) A look into stem cell therapy:
Exploring the options for treatment of ischemic stroke. Stem Cells Int.
84.
Tsang KS, Ng
CPS, Zhu XL, Wong GKC, Lu G, et al. (2017) Phase I/II randomized controlled
trial of autologous bone marrow-derived mesenchymal stem cell therapy for
chronic stroke. World J Stem Cells 9: 133-143.
85.
Duan X, Lu L,
Wang Y, Zhang F, Mao J, et al. (2017) The long-term fate of mesenchymal stem
cells labeled with magnetic resonance imaging-visible polymersomes in cerebral
ischemia. Int J Nanomed 12: 6705-6719.
86.
Seo HG, Yi Y, Oh BM, Paik NJ (2017) Neuroprotective effect
of secreted factors from human adipose stem
cells in a rat stroke model. Neurol Res 39: 1114-1124.
87.
Wei L, Wei ZZ,
Jiang MQ, Mohamad O, Yu SP (2017) Stem cell transplantation therapy for
multifaceted therapeutic benefits after stroke. Prog Neurobiol 157: 49-78.
88.
Chen L, Qiu R,
Li L, He D, Lv H, et al. (2014) The role of exogenous neural stem cells
transplantation in cerebral ischemic stroke. J Biomed Nanotechnol 10:
3219-3230.
89.
Cox CS (2017) Cellular therapy for traumatic neurological injury. Pediatr
Res.
90.
Longhi L, Zanier ER, Royo N, Stochetti N, McIntosh TK (2005) Stem cell
transplantation as a therapeutic strategy for traumatic brain injury. Transpl
Immunol 15: 143-148.
91.
Chen Q, Long Y, Yuan X, Zou L, Sun J, et al. (2005) Protective effects of
bone marrow stromal cell transplantation in injured rodent brain: Synthesis of
neurotrophic factors. J Neurosci Res 80: 611-619.
92.
Xue S, Zhang HT, Zhang P, Luo J, Chen ZZ, et al. (2010) Functional
endothelial progenitor cells derived from adipose tissue show beneficial effect
on cell therapy of traumatic brain injury. Neurosci Lett 473: 186-191.
93.
Walker PA, Harting MT, Jimenez F, Shah SK, Pati S, et al. (2010) Direct
intrathecal implantation of mesenchymal stromal cells leads to enhanced
neuroprotection via an NFkappaB-mediated increase in interleukin-6 production.
Stem Cells Dev 19: 867-876.
94.
Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, et al. (2011)
Mesenchymal stem cell therapy modulates the inflammatory response in
experimental traumatic brain injury. Neurol Res Int 2011: 564089.
95.
Grigorian AS, Gilerovich EG, Pavlichenko NN, Kruglyakov PV, Sokolova IB,
et al. (2011) Effect of transplantation of mesenchymal stem cells on neuronal
survival and formation of a glial scar in the brain of rats with severe traumatic
brain injury. Bull Exp Biol Med 150: 551-555.
96.
Lundberg J, Sodersten E, Sundstrom E, Le Blanc K, Andersson T, et al.
(2012) Targeted intra-arterial transplantation of stem cells to the injured CNS
is more effective than intravenous administration: Engraftment is dependent on
cell type and adhesion molecule expression. Cell Transplant 21: 333-343.
97.
Jiang J, Bu X, Liu M, Cheng P (2012) Transplantation of autologous bone
marrow-derived mesenchymal stem cells for traumatic brain injury. Neural
Regen Res 5: 46-53.
98.
Zhang R, Liu Y, Yan K, Chen L, Chen XR, et al. (2013) Anti-inflammatory
and immunomodulatory mechanisms of mesenchymal stem cell transplantation in
experimental traumatic brain injury. J Neuroinflammation 10: 106.
99.
Anbari F, Khalili MA, Bahrami AR, Khoradmehr A, Sadeghian F, et al.
(2014) Intravenous transplantation of bone marrow mesenchymal stem cells promotes
neural regeneration after traumatic brain injury. Neural Regen Res 9: 919-923.
100.Peng W, Sun J, Sheng C, Wang
Z, Wang Y, et al. (2015) Systematic review and meta-analysis of efficacy
of mesenchymal stem cells on locomotor recovery in animal models of traumatic
brain injury. Stem Cell Res Ther 6: 47.
101.Turtzo LC, Budde MD, Dean
DD, Gold EM, Lewis BK, et al. (2015) Failure of intravenous or intracardiac
delivery of mesenchymal stromal cells to improve outcomes after focal traumatic
brain injury in the female rat. PLoS One 10: e0126551.
102.Mishra SK, Rana P, Khushu
S, Gangenahalli G (2017) Therapeutic prospective of infused allogenic cultured mesenchymal
stem cells in traumatic brain injury mice: A longitudinal proton magnetic
resonance spectroscopy assessment. Stem Cells Transl Med 6: 316-329.
103.Dori I, Petrakis S, Giannakopoulou
A, Bekiari C, Grivas I, et al. (2017) Seven days post-injury fate and effects
of genetically labelled adipose-derived mesenchymal cells on a rat traumatic
brain injury experimental model. Histol Histopathol 32: 1041-1055.
104.Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, et al. (2017)
Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol
8: 28.
105.Tian C, Wang X, Wang X, Wang L, Wang X, et al. (2013) Autologous bone
marrow mesenchymal stem cell
therapy in the subacute stage of traumatic
brain injury by lumbar puncture.
Exp Clin Transplant 11: 176-181.
106.Liao GP, Harting MT, Hetz RA,
Walker PA, Shah SK, et al. (2015) Autologous bone marrow mononuclear cells reduce
therapeutic intensity for severe traumatic brain injury in children. Pediatr
Crit Care Med 16: 245-255.
107.Vaquero J, Zurita M, Bonilla
C, Fernández C, Rubio JJ, et al. (2017) Progressive increase in brain glucose
metabolism after intrathecal administration of autologous mesenchymal stromal
cells in patients with diffuse axonal injury. Cytotherapy 19: 88-94.
108.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, et al. (1999) Autologous
transplantation of bone marrow cells improves damaged heart function.
Circulation 100: II247-256.
109.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, et al. (2003)
Adult cardiac stem cells are multipotent and support myocardial regeneration.
Cell 114: 763-776.
110.Torella D, Ellison GM, Méndez-Ferrer
S, Ibañez B, Nadal-Ginard B (2006) Resident human cardiac stem cells: Role in
cardiac cellular homeostasis and potential for myocardial regeneration. Nat
Clin Pract Cardiovasc Med Suppl 1: S8-13.
111.Barile L, Messina E,
Giacomello A, Marbán E (2007) Endogenous cardiac stem cells. Prog Cardiovasc
Dis 50: 31-48.
112.Noiseux N, Gnecchi M, Lopez-Ilasaca
M, Zhang L, Solomon SD, et al. (2006) Mesenchymal stem cells overexpressing Akt
dramatically repair infarcted myocardium and improve cardiac function despite
infrequent cellular fusion or differentiation. Mol Ther 14: 640-650.
113.Costoya JA, Finidori J, Moutoussamy
S, Senarís R, Devesa J, et al. (1999) Activation of growth hormone receptor
delivers an antiapoptotic signal: Evidence for a role of Akt in this pathway.
Endocrinology 140: 5937-5943.
114.Xu JY, Liu D, Zhong Y, Huang RC (2017) Effects of timing on
intracoronary autologous bone marrow-derived cell transplantation in acute
myocardial infarction: A meta-analysis of randomized controlled trials. Stem
Cell Res Ther 8: 231.
115.Xu JY, Cai WY, Tian M, Liu D, Huang RC (2016) Stem cell
transplantation dose in patients with acute myocardial infarction: A
meta-analysis. Chronic Dis Transl Med 2: 92-101.
116.Bianconi V, Sahebkar A, Kovanen P, Bagaglia F, Ricciuti B, et al.
(2017) Endothelial and cardiac progenitor cells for cardiovascular repair: A
controversial paradigm in cell therapy. Pharmacol Ther pii:
S0163-7258(17)30214-0.
117.Bayes-Genis A, Soler-Botija C, Farré J, Sepúlveda P, Raya A, et al.
(2010) Human progenitor cells derived from cardiac adipose tissue ameliorate
myocardial infarction in rodents. J Mol Cell Cardio l49: 771-780.
118.Roura S, Gálvez-Montón C, Mirabel C, Vives J, et al. (2017)
Mesenchymal stem cells for cardiac repair: Are the actors ready for the
clinical scenario? Stem Cell Res Ther 8: 238.
119.Sacca L, Cittadini A, Fazio S (1994) Growth hormone and the heart. Endocr Rev 15: 555-573.
120.Li Q, Li B, Wang X, Leri A, Jana KP, et al. (1997) Overexpression of
insulin-like growth factor-I in mice protects from myocyte death after
infarction, attenuating ventricular dilation, wall stress and cardiac
hypertrophy. J Clin Invest 100:
1991-1999.
121.Timsit J, Riou B, Bertherat J, Wisnewsky C, Kato NS, et al. (1990)
Effects of chronic growth hormone hypersecretion on intrinsic contractility,
energetics, isomyosin pattern and myosin adenosinetriphosphatase activity of
ratleft ventricle. J Clin Invest
86: 507-515.
122.Cittadini A, Ishiguro Y, Stromer H, Spindler M, Moses AC, et al.
(1998) Insulin-like growth factor-1 but not growth hormone augments mammalian
myocardial contractility by sensitizing the myofilament to Ca2+
through a wortmannin-sensitive pathway: Studies in rat and ferret isolated
muscles. Circ Res 83: 50-59.
123.Mayoux E, Ventura-Clapier R, Timsit J, Behar-Cohen F, Hoffmann C, et
al. (1993) Mechanical properties of rat cardiac skinned fibers are altered by
chronic growth hormone hypersecretion. Circ
Res 72: 57-64.
124.Bruel A, Oxlund H (1995) Biosynthetic growth hormone increase the
collagen deposition rate in rat aorta and heart. Eur J Endocrinol 132: 195-199.
125.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, et al. (1995)
Cardioprotective effect of insulin-like growth factor I in myocardial ischemia
followed by reperfusion. Proc Natl
Acad Sci U S A 92: 8031-8035.
126.Ren J (2002) Short-term administration of insulin-like growth factor
(IGF-1) does not induce myocardial IGF-1 resistance. Growth Horm IGF Res 12: 162-168.
127.Kusano K, Tsutsumi Y, Dean J, Gavin M, Ma H, et al. (2007) Long-term
stable expression of human growth hormone by rAAV promotes myocardial
protection post-myocardial infarction. J
Mol Cell Cardiol 42: 390-399.
128.Rong SL, Lu YX, Liao YH, Wang XL, Wang YJ, et al. (2008) Effects of
transplanted myoblasts transfected with human growth hormone gene on
improvement of ventricular function of rats. Chin Med J 121: 347-354.
129.Nakajima K, Fujita J, Matsui M, Tohyama S, Tamura N, et al. (2015)
Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and
angiogenesis to ameliorate cardiac function after myocardial infarction. PLos
One 10: e0133308.
130.Helie A, Brinker T (2011) Clinical translation of stem cell therapy in
traumatic brain inury: The potential of encapsulated mesenchymal cell
biodelivery of glucagon-like peptide-1. Dialogues Clin Neurosci 13: 279-286.
131.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, et al. (2016)
Intravenous administration of xenogenic adipose-derived mesenchymal stem cells
(ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and
preserved neurological function in rat after acute ischemic stroke. Oncotarget
7: 74537-74556.
132.Bang OY, Kim BH, Cha JM, Moon GJ (2016) Adult stem cell therapy for
stroke: Challenges and progress. J Stroke 18: 256-266.
133.Chung CY, Lin MH, Lee IN, Lee TH, Lee MH, et al. (2017) Brain-derived
neurotrophic factor loaded PS80 PBCA nanocarrier for in vitro neural differentiation of mouse induced pluripotent stem cells.
Int J Mol Sci 18: E663.
134.Wang TW, Chang KC, Chen LH, Liao SY, Yeh CW, et al. (2017) Effects of
an injectable functionalized self-assembling nanopeptide hydrogel on
angiogenesis and neurogenesis for regeneration of the central nervous system.
Nanoscale 9: 16281-16292.
135.Shichinohe H, Kawabori
M, Iijima H, Teramoto T, Abumiya T, et al. (2017) Research on advanced intervention using novel bone marrow stem cell
(RAINBOW): A study protocol for a phase I, open-label, uncontrolled,
dose-response trial of autologous bone marrow stromal cell transplantation in
patients with acute ischemic stroke. BMC Neurol 17: 179.
136.Tseng KY, Anttila JE, Khodosevich K, Tuominen RK, Lindahl M, et al.
(2017) MANF promotes differentiation and migration of neural progenitor cells with
potential neural regenerative effects in stroke. Mol Ther.
137.Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2016) Stem cell
therapy for chronic ischaemic heart disease and congestive heart failure.
Cochrane Database Syst Rev 2: CD007888.
138.Lewis FC, Kumar SD, Ellison-Hughes GM (2017) Non-invasive strategies
for stimulating endogenous repair and regenerative mechanisms in the damaged
heart. Pharmacol Res.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Spine Diseases
- Journal of Alcoholism Clinical Research