727
Views & Citations10
Likes & Shares
Background: This study was designed to prospectively
evaluate the changes in tissue Doppler imaging (TDI) at mitral and tricuspid
annuli in patients undergoing pericardiectomy for chronic constrictive
pericarditis and identify the relationship if any of the tissue Doppler
imaging-derived variables with patient’s symptomatic status following surgery.
Patients and methods: Fifty-four patients undergoing
pericardiectomy for constrictive pericarditis aged 7 years to 70 years (mean
31.0 ± 16.8 years) were studied for 24.4 ± 10.8 months (range 6-42 months).
They underwent Doppler flow velocity and TDI studies. Generalized estimating
equation was used to test the changes in TDI-derived mitral and tricuspid
annular velocities in postoperative period from baseline.
Results: Despite congestive heart failure, all
patients had normal left ventricular ejection fraction and increased medial
mitral and tricuspid early diastolic septal velocity (e¢) with “annulus reversus”. This pattern of
annular velocity improved maximally in the immediate postoperative period. At
closing interval, 10 (18.5%) patients continued to be in New York Heart
Association class II and 9 of them continued to remain in atrial fibrillation.
There were no differences of TDI-derived systolic and diastolic annular velocities
of the mitral and tricuspid valves in the preoperative period between
symptomatic and asymptomatic patients.
Conclusion: We conclude that preoperative atrial
fibrillation is a predictor of poor prognostic outcome following
pericardiectomy. Tissue Doppler imaging-derived mitral and tricuspid annular
velocities are non-predictors of postoperative outcome following
pericardiectomy. Tissue Doppler imaging is a useful investigative modality for
diagnosis of constrictive pericarditis and not a useful indicator for
postoperative evaluation.
Keywords: Tissue Doppler imaging, Chronic constrictive
pericarditis, Pericardiectomy, Echocardiography
INTRODUCTION
Doppler myocardial
imaging is an echocardiographic technique that has the potential to enhance
diagnostic information available from Doppler blood-flow indices [7-11].
Specifically, tissue Doppler imaging (TDI) has allowed the determination of
discrete amplitude cut-off points at the lateral mitral annulus to distinguish
CP from RCM without overlap [7,8].
Because the mechanoelastic properties of the
myocardium are preserved in CP, the longitudinal mitral annular velocities are
normal. Tissue Doppler imaging can measure mitral or tricuspid annular motion
which reflects ventricular systolic and diastolic motion in the long axis
[7-10]. In constrictive pericarditis, early diastolic septal velocity (medial e¢) is preserved or even increased [12,13], due to limitation of lateral
expansion by the constricting pericardium, and early diastolic lateral mitral
annular velocity (mitral lateral e¢) tends to be lower
than medial e¢ which is a
reversal of their normal relationship [7,13-15]. This mitral annular velocity
pattern is relatively specific for CP in patients with heart failure, since e¢ velocity is usually reduced in patients with myocardial disease whether
left ventricular ejection fraction (LVEF) is preserved or reduced. However,
there are limited data on mitral and tricuspid annular velocities in patients
with CP and their changes after pericardiectomy [12,16,17]. Furthermore, these
publications have not addressed the degree and timing of reduction of these
annular velocities and their relationship with the patient’s symptomatic status
following surgery.
This prospective non-randomized study aims to:
a. Serially evaluate the immediate and late effects of
total pericardiectomy on the clinical outcome and left ventricular size and
function.
b. Serially assess the effect of total pericardiectomy
on mitral and tricuspid diastolic filling velocities and their respiratory
variation.
c.
Serially
assess the effect of pericardiectomy on mitral and tricuspid lateral and medial
systolic and diastolic annular velocities.
d. analyze the relationship if any of the mitral and
tricuspid annular velocities with global myocardial function before and after
total pericardiectomy and,
e.
Analyze the
relationship of mitral and tricuspid annular velocities with patient’s
symptomatic status in the pre- and postoperative period.
PATIENTS
AND METHODS
Patients were enrolled for this prospective study following institutional ethics committee approval and informed written consent from patients/guardians. Between June 2013 and December 2016, 54 consecutive patients (41 males) undergoing pericardiectomy for chronic constrictive pericarditis at All India Institute of Medical Sciences, New Delhi, operated by a single surgeon (corresponding author) were included in this prospective study. The decision to perform pericardiectomy was based on clinical, echocardiographic, computed tomographic and/or cardiac catheterization criteria. Patients with clinical, operative and pathological features of pericarditis and constriction were included. Patients undergoing creation of pleuropericardial window for pericardial effusion, pericardial biopsy and concomitant pericardiectomy and repair of congenital or acquired heart diseases were excluded. Descriptive characteristics and relevant details are summarized in Table 1. Patients age at operation ranged from 7 to 70 years (mean, 31 ± 16.8 years). Duration of symptoms ranged from 8 months to 5 years (mean, 18.4 ± 12.6). Preoperatively, 30 (55.6%) patients and 24 (44.4%) patients were in New York Heart Association (NYHA)-III and IV, respectively. All patients had congestive heart failure as the predominant symptom in the preoperative period. Forty-eight (88.8%) patients had precordial pain, 3 (5.5%) had evidence of cardiac tamponade and atrial fibrillation was found in 26 (48.1%) patients. Ninety-two percent had distended jugular veins, 83% ascites, 79% hepatomegaly, 41% pleural effusion and 17% had pulsus paradoxes.
Four out of 54 patients with pericardial effusion
required tapping and steroid therapy as appropriate. All patients with
tuberculosis (n=40, 74%) received multidrug therapy (isoniazid, rifampicin,
ethambutol and pyrazinamide) for 3 months followed by triple-drug therapy for 9
months after operation. Preoperatively, all patients were on digitalis and
diuretics.
The etiology was considered tubercular if the
histopathology of the excised pericardium showed granulomas, caseation, giant
cells (n=34, 62.9%) or if the fluid and debris removed at surgery was positive
for acid fast bacilli (n=6, 18.5%). A history of pulmonary and lymph node
tuberculosis was present in 10 (18.5%) and 4 (7.4%) patients respectively.
Fourteen (26%) patients had pyogenic or effusive-constrictive pericarditis not
resolving with pericardiocentesis.
Laboratory investigations showed elevated
erythrocyte sedimentation rate (range, 40 to 90 mm at 1 h) in 19 (35.1%), renal
dysfunction (serum creatinine>2 mg/dl in 14 (25.9%)) and hyperbilirubinemia
in 19 (35.1%) patients. Chest roentgenogram revealed pericardial calcification
(n=20, 37%), pleural effusion (n=22, 40.7%) and pulmonary infiltrates (n=9;
16.6%) patients. The calcification was distributed over the anterior and
inferior surfaces of the heart in 12 (22.2%) patients and all around the heart
like a cocoon in 8 (14.8%) patients. None had mitral annular calcification.
Electrocardiogram revealed low voltage QRS complex (n=53, 98.1%), flattening or
T-wave inversion (n=49, 90.7%), atrial fibrillation (n=26, 48.1%) and premature
ventricular complex (n=8, 14.8%). Twenty of twenty-six (76.9%) with atrial
fibrillation were in NYHA class-IV.
Echocardiography revealed pericardial thickness
(>4 mm, n=54), inferior vena cava dilatation (n=53), right atrial
enlargement (n=53), abnormal septal motion (n=52), >25% increase in mitral
inflow velocity with expiration compared with inspiratory phase (n=53),
moderate mitral regurgitation (grade 2+, n=8) and moderate tricuspid
regurgitation (grade 2, n=8). Preoperative cardiac catheterization was
performed in 6 patients. The rest did not have catheterization because of their
class III and IV symptoms with hepatic dysfunction, renal dysfunction or the
echocardiographic findings were unequivocal. All demonstrated the findings
consistent with constrictive pericarditis because of an elevated right atrial
pressure, usually with a M- or W-shaped contour, an abnormally high right
ventricular end-diastolic pressure with a characteristic dip-plateau diastolic
configuration, equalization of end-diastolic pressure in all cardiac chambers
and a ratio or right ventricular end-diastolic-to-right ventricular systolic
pressure of >0.30.
SURGICAL
TECHNIQUES
The surgical approach was based on surgeon
preference and remained uniform throughout the study period. However, a left
anterolateral thoracotomy was the preferred option in the setting of purulent
pericarditis to avoid sternal infection. The median sternotomy approach was
preferred in the following cases: (1) Annular constrictive pericarditis, (n=9,
16.6%); (2) Calcific pericardial patch compressing the right atrium and right
ventricular outflow tract (n=12, 22.2%); (3) Egg shaped calcified pericardium
(n=8, 14.8%) and (4) extra cardiac intrapericardial mass (n=3, 5.5%). One patient
required institution of cardiopulmonary bypass to control bleeding from right
ventricular outflow tract. The detailed operative steps of pericardiectomy via
median sternotomy (n=34) and left anterolateral thoracotomy (n=20) have been
addressed in our previous publications [4,5].
In patients with gross ascites, a peritoneal
drainage catheter was placed in the peritoneal cavity before surgical incision
and was kept on continuous drainage. It was removed after 1 or 2 days in
intensive care unit depending upon the drainage amount. Surgical manipulation
of the heart during pericardiectomy can make thermo dilution calculation and
pulmonary artery pressure monitoring unreliable as monitors and hence was not
used in this group of patients.
After sternotomy, the thymus and pleural reflections
were mobilized laterally to obtain a wide width of the pericardium. Both
pleural cavities were widely opened to remove the pleural fluid and to identify
the phrenic pedicles on either side [4].
An I-shaped incision was made in the midline over
the pericardium up to the level of the pulmonary artery superiorly and
diaphragm inferiorly. The dissection of the pericardium off the heart was done
using cautery until the fibrous pericardium along with its serous layer. When
it was done properly, there was clear visualization of the epicardial fat and
the coronary arteries. Inability to visualize the coronaries indicates that the
dissection plane was not deep enough.
The cautery was adjusted between 8-10 mV during the
process of dissection to avoid cautery induced ventricular fibrillation.
Multiple silk stay sutures were then placed on the cut edges of the incised
pericardium. The pericardium was initially divided at the bottom portion close
to the diaphragmatic reflection over the right ventricle and the lateral
pericardial flap was raised superiorly and laterally. Circumferential patches
of calcified pericardium were crushed with a thick hemostat and/or bone cutter
and were removed avoiding injury to the underlying vascular structures,
coronaries and phrenic nerves. We have not used cavitational ultrasonic
surgical aspiration system for removal of calcium or nerve stimulator for
identification of the phrenic nerve on any patient in this study.
The pericardium covering ventricles, the great
vessels, the venae cava and the right atrium was excised 1 cm anterior to the
phrenic nerve on either side. The pericardium over the venae cava and right
atrium was resected last. The pericardial and pleural cavities were irrigated
with normal saline.
For anterolateral thoracotomy, patients were
positioned in left lateral position with groin exposed and prepared [5]. The
left anterolateral thoracotomy was carried out through left fourth intercostal
space. After entering the left thoracic cavity, pleural reflection was dissected
out from pericardium. Anteriorly, the pericardium was mobilised and adhesions
between sternum and pericardium was released. This was followed by two
full-length parallel incisions 0.5 cm anterior and posterior to the left
phrenic nerve and extended until the level of the pulmonary artery superiorly
and the diaphragm inferiorly. Multiple silk stay sutures were placed on the
incised edges of the pericardium to achieve adequate exposure. Posteriorly, the
pericardium was gently dissected from the posterolateral surface of the left
ventricle and left atrial appendage. The posterior pericardium was subsequently
divided to facilitate adequate mobilization until the levels of left-sided
pulmonary veins and excised. The anterior pericardial flap was held between stay
sutures and mobilized from the anterolateral surfaces of right ventricle, right
ventricular outflow tract, and pulmonary artery. Using cautery, a new cleavage
plane was made to develop between the diaphragm and thickened diaphragmatic
pericardium all along its length. The diaphragmatic surface of the right
ventricle and the left ventricular apex was completely freed from pericardial
adhesions. Subsequently, the entire width of diaphragmatic pericardium was
excised in toto.
ECHOCARDIOGRAPHIC
STUDIES AND MEASUREMENTS
All patients had comprehensive evaluation with
M-mode, two-dimensional (2D) and pulsed-wave Doppler echocardiography with a
respirometer recording and tissue Doppler imaging (TDI) before and after
pericardiectomy using a Phillips iE 33 with 2.0 to 5.0 MHz transducer. Left
ventricular ejection fraction (LVEF) was calculated by 2D echocardiography with
a modification of the method of Quinones and colleagues [18]. Left atrial
volume was measured by the modified biplane area-length method [19]. Right
ventricular systolic function was visually assessed. By using pulsed wave
Doppler echocardiography, the following variables were measured: trans-mitral
and trans-tricuspid peak velocities of early (E) and late filling (A) and E
wave deceleration time (DT). On TDI, peak annular velocities were measured from
the apical four chamber view at systole (s'), early (e') and late (a') diastole
with a 2-5 mm tissue Doppler sample volume placed at the septal corner and at
the mitral and tricuspid lateral annuli. In patients with atrial fibrillation,
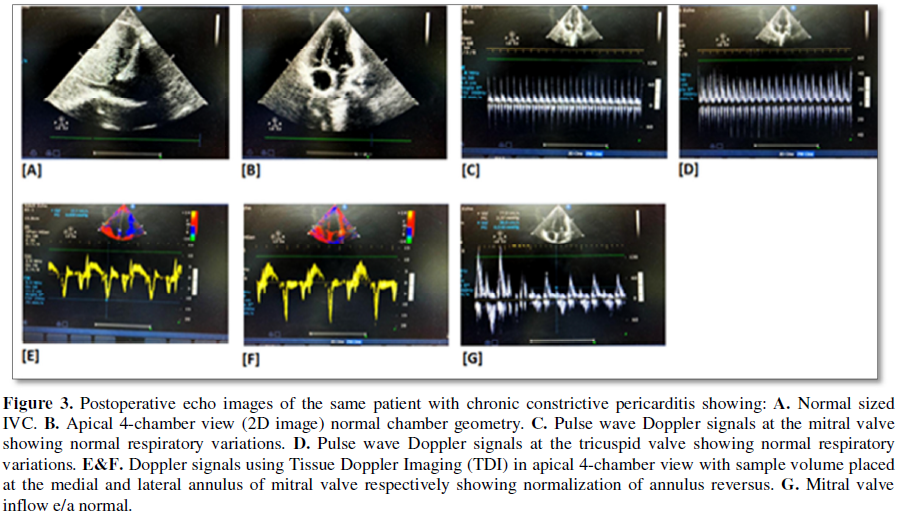
five consecutive signals were measured and averaged. Inferior vena caval (IVC)
diameter was assessed in subcostal sagittal view.
POSTOPERATIVE
STUDIES
These included 3-monthly clinical examinations,
electrocardiogram and chest radiographs. A minimum of 6 months follow-up was
mandatory for this study. Preoperative studies were performed within 7 days
before surgery. Postoperatively, all survivors were followed
echocardiographically at the time of discharge and at 6 months. All late echoes
have been grouped into one time period (6 months) with a range of no greater
than 6 months. Echocardiographic data were measured according to American
Society of echocardiographic criteria [20].
Definitions
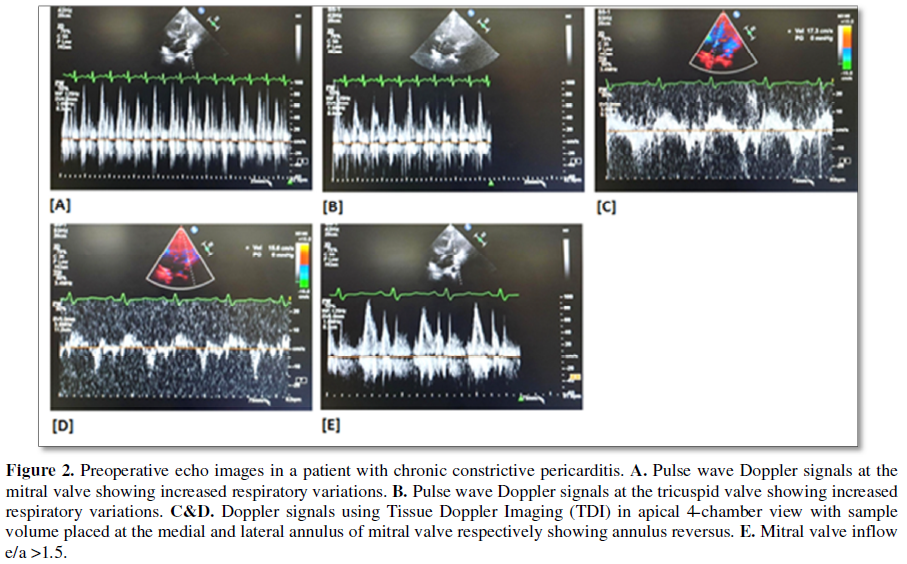
On Doppler, two flow velocity envelopes can be seen
during diastole in persons with sinus rhythm: the E-wave, representing the
early, passive filling of the ventricle, and the A-wave, that happens late in
diastole, representing the active filling, the atrial contraction. For both
mitral and tricuspid valve E and A wave measured. Mitral or tricuspid
regurgitation was assessed semi-quantitatively as grade 1+ to 4+. A
constrictive pattern was defined as 25% or greater increase in mitral
E-velocity with expiration as compared with inspiration and an augmented (25%
or more) diastolic flow reversal in the hepatic vein after the onset of
expiration compared with inspiration. On tissue Doppler imaging, lateral mitral
e¢, represents early diastolic myocardial
relaxation velocity below the baseline as the annulus ascends away from the
apex with cursor at lateral annulus; medial mitral e¢ and
lateral tricuspid e¢ are same
velocities measured at mitral medial annulus and tricuspid lateral annulus
respectively. The mitral lateral s¢ velocity
represents the systolic myocardial velocity at lateral mitral annulus. The
medial mitral s¢ and lateral
tricuspid s¢ are same velocities measured at mitral
medial annulus and tricuspid lateral annulus, respectively.
For uniformity with other studies, total pericardiectomy
was defined as wide excision of the pericardium with the phrenic nerves
defining the posterior extent, the great vessels including the intrapericardial
portion of superior vena cava and superior vena cava- right atrial junction
defining the superior extent, and the diaphragmatic surface, including the
inferior vena cava- right atrial junction defining the inferior extent of the
pericardial resection [4]. Constricting layers of the epicardium were removed
whenever possible. The atria and venae cava were decorticated as a routine in
all cases in this study group. Pericardiectomy was considered partial if both
ventricles could not be decorticated completely because of dense myopericardial
adhesions or calcification.
Constrictive pericarditis was considered to be
hemodynamically significant when there were clinical features of constriction
with supportive echocardiographic and hemodynamic criteria as outlined earlier.
Perioperative mortality was defined as that occurring within 30 days after surgery.
Cardiac-related death was defined as death due to cardiac causes, such as
progressive congestive cardiac failure [6-10]. Hypoproteinemia was defined as
serum albumin level <3.5 g/dl. Renal dysfunction was defined as serum
creatinine >2.0 g/dl.
Low output syndrome was diagnosed if the patient
required inotropic support (dopamine (4-10 µg.kg-1.min-1),
dobutamine (5-10 µg.kg-1.min-1), epinephrine (0.01-0.1
µg.kg-1.min-1), milrinone (50 µg/kg intravenous bolus
followed by 0.375-0.75 µg.kg-1.min-1)), either isolated
or in combination, in the operating room or in the intensive care unit to
maintain stable hemodynamics in the absence of mechanical external compression
after correction of all electrolytes or blood gas abnormalities and after
adjusting the preload to its optimal value. Low output syndrome was also
diagnosed if there was an increasing requirement of the above-mentioned
inotropes with or without intra-aortic balloon counter pulsation along with
afterload reduction with sodium nitroprusside. Patients who received less than
4 µg.kg-1.min-1 of dopamine to increase renal perfusion
were not considered to have low output syndrome.
Accordingly, under the definition of low output
syndrome after pericardiectomy, an integration of relevant clinical, laboratory
and bedside echocardiographic criteria were used. The criteria for diagnosis
were as follows: cold extremities, absent pedal pulses, decreased toe
temperature, reduced systolic pressure, impaired renal function and oliguria
(<1.0 ml.kg-1.h-1), metabolic acidosis, increased
serum lactate levels > 2.0 mmol/L, >2 h), low mixed venous oxygen
saturation (<50%) and blunt sensorium.
STATISTICAL
ANALYSIS
Statistical analysis was carried out using Stata
11.0 (College Station, Texas, USA). Continuous data were presented as mean ±
standard deviation, whereas categorical variables were presented as frequency
distribution and percentage. Qualitative data were analysed by using c2 test or student’s t test. Normality
assumptions for continuous variables were assessed using Shapiro-Wilks test.
Comparisons between two groups were done with the t-test. Echocardiographic
parameters over a period of time between various clinical parameters were
tested using generalized estimating equation with exchangeable correlation
analysis. The correlation between mitral annular systolic velocities and left
ventricular ejection fraction was assessed using Spearman’s rank correlation.
The p value of <0.05 was considered as statistically significant.
RESULTS
There was no early death. Fifty patients had
low-cardiac-output in the immediate postoperative period. All patients were
routinely started on dopamine (4 µg.kg-1.min-1) to
increase renal perfusion on operation table after completing excision of the
thickened pericardium. Patients with normal renal function were administered
oral angiotensin-converting enzyme (ACE) inhibitors before weaning from
inotropic agents. Postoperatively, digoxin, diuretics and ACE-inhibitors were
weaned at varying time intervals.
Patients considered to have low output syndrome
(n=50) required dopamine (4-10 µg.kg-1.min-1),
epinephrine (0.01-0.1 µg.kg-1.min-1) and milrinone (50
µg/kg intravenous bolus followed by 0.375-0.75 µg.kg-1.min-1)
either isolated or in combination. Median duration of inotrope requirement was
4 days (range 2-7 days) in these patients. Patients with normal renal function
were administered oral angiotensin-converting enzyme inhibitors before weaning
from inotropic agents. Two patients required intraoartic balloon counter
pulsation as an additional support. There was marked reduction of filling
pressure within 24 h in the majority of patients (n=44) after total
pericardiectomy (mean=right atrial pressure (RAP) 16.72 ± 4.0 (7-26) to 9.11 ±
0.96 (7-10); p<0.001) (Table 1). Echocardiographically, diastolic
filling characteristics remained abnormal in 19 (35.2%) patients of the study
group in the immediate postoperative period. There was no late death.
Reoperation was not required for any patients.
Follow-up
Follow-up was 100% complete (range 6-48 months,
median 28) and yielded 135.9 patients-years of data with a mean follow-up time
of 30.2 ± 10.8 months.
At closing interval, 10 (18.5%) patients continued
to remain in NYHA class II and had persistent abnormalities of the diastolic
filling pattern (p<0.05) on Doppler echocardiography. Pairwise comparison
between symptomatic (n=10, 18.5%) and asymptomatic (n=44, 81.5%) patients
revealed significant abnormality of the indexed IVC diameter (p<0.05) and
increased left ventricular end-diastolic internal diameter (LVID) (p<0.05)
in all patients of the symptomatic group. Nine of these symptomatic patients
continued to remain in atrial fibrillation. Preoperatively, these symptomatic patients
(n=10) were in NYHA class IV and were in atrial fibrillation. Thus, 9 (34.6%)
of 26 patients who had preoperative atrial fibrillation continued to remain in
atrial fibrillation. This could be the causative factor for alteration of left
atrial mechanics and the left ventricular filling pressure which could lead to
ongoing symptoms. Surgical techniques did not affect the outcome of atrial
fibrillation.
These symptomatic patients (n=10, 18.5%) had
significantly higher right atrial pressure in the immediate preoperative period
compared to the asymptomatic group (n=44, 81.5%) (Mean RAP=20.6 ± 3.6
(symptomatic) vs. 16.72 ± 4 mm Hg (asymptomatic), p<0.05). Postoperatively,
despite total pericardiectomy, the right atrial pressure of the symptomatic
group continued to remain higher than the asymptomatic group (mean RAP=13.80 ±
3.17 (symptomatic) vs. 9.11 ± 0.96 mm Hg (asymptomatic), p<0.001)). There
were no differences of TDI-derived systolic and diastolic annular velocities of
the mitral and tricuspid valves between symptomatic and asymptomatic patients
in the preoperative period (Tables 2 and 3). Tissue Doppler
imaging-derived mitral and tricuspid annular velocities failed to predict the
postoperative outcome of patients undergoing pericardiectomy (Tables 2 and 3).
Data
analyses and study interpretation of echocardiographic data (Tables 4 and 5 and
Figures 1-3)
To assess the characterization of the mitral and
tricuspid annular velocity changes in patients undergoing pericardiectomy for
constructive pericarditis, generalized estimating equation analysis revealed
the following results:
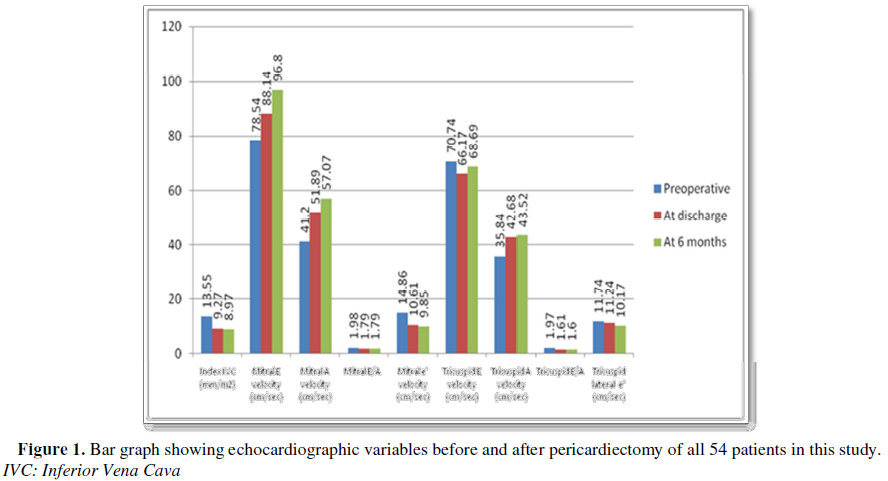
1. There was statistically significant reduction in
indexed IVC diameter in the immediate (p<0.001) as well as late
postoperative period (p<0.001). The indexed IVC diameter decreased from a
preoperative value of 13.55 ± 2.58 mm/m2 to 9.27 ± 2.47 mm/m2
(at discharge) and 8.97 ± 2.78 mm/m2 (at 6 months follow-up).
2. Doppler flow velocity envelopes revealed
statistically significant improvement of both transmitral early diastolic and
late diastolic filling velocities in the immediate as well as late
postoperative period. As a result, the mitral valve E/A also improved from 1.98
± 0.39 (preoperative) to 1.79 ± 0.45; p<0.05 (immediate postoperative) and
1.79 ± 0.47; p<0.05 (late postoperative).
3. There was insignificant change in trans-tricuspid
early diastolic filling velocity; however, there was significant improvement of
trans-tricuspid late diastolic filling velocity secondary to atrial
contraction. Overall, the tricuspid valve E/A improved from a preoperative
level of 1.97 ± 0.33 to 1.61 ± 0.35 (p<0.001) at discharge and 1.60 ± 0.40
(p<0.001) in the late postoperative period.
4. All patients demonstrated the classic phenomenon of
“annulus reversus” of mitral valve velocities. Following pericardiectomy, in
the immediate postoperative period, there was no statistically significant
improvement of mitral lateral e¢ velocity; however there was statistically
significant improvement of mitral lateral e¢ velocity at 6
months following pericardiectomy (p=0.001). All patients demonstrated greater
significant reduction of medial e¢ velocity following pericardiectomy in both
immediate and late postoperative period.
5. The lateral and medial e¢ velocity of
the tricuspid valve also exhibited similar phenomenon. Both medial and lateral
tricuspid annular velocities exhibited statistically significant decrease in
the late postoperative period and only medial tricuspid annular velocity
exhibited significant decrease in the immediate postoperative period.
6. Preoperatively, all patients exhibited an
inspiratory decrease in peak transmitral flow (mean E>29.76 ± 8.69%) and an
increased transtricuspid flow (mean E>30.55% ± 7.81%).
7. Following pericardiectomy, all patients demonstrated
statistically significant reduction in mitral systolic annular velocity
(lateral and medial) in both early and late postoperative period (8.72 ± 1.5
cm/s (preoperative) vs. 7.94 ± 1.82 cm/s (immediate postoperative); p=0.001 and
7.08 ± 1.20 cm/s (late postoperative); p<0.001; systolic medial mitral
annular velocity (mitral medial s¢) 7.77 ± 1.45 cm/s (preoperative) vs. 7.15 ± 1.45
cm/s (early postoperative) and 6.22 ± 1.09; p<0.0001). The correlation
between mitral s¢ and LVEF was statistically insignificant.
8. Similarly, following pericardiectomy, all patients
demonstrated statistically significant reduction in tricuspid lateral annular
systolic velocity in both early and late postoperative period [tricuspid s¢ (cm/s) 9.12 ± 1.96 (preoperative) vs. 8.20 ± 1.73 (early postoperative);
p<0.05 vs. 7.16 ± 1.80 cm/s (late postoperative); p<0.001).
9. The early postoperative left ventricular
end-diastolic internal diameter (LVID), left ventricular end-systolic internal
diameter (LVIS) and LVEF remained almost same as compared to preoperative
measurements. There were no significant changes of the above variables in late
postoperative period.
10. Overall, the degree of changes of Doppler and
TDI-derived variables was maximal in the immediate postoperative period.
DISCUSSION
So far as we are aware, there have been few
published studies in the literature investigating the role of tissue Doppler
imaging-derived parameters of mitral and tricuspid annular motion on global and
regional ventricular function and their role in differentiating CP from RCM
[7-17].
The principal findings of this investigation
include:
1. Significant reduction in indexed IVC diameter and
significant improvement of early and late diastolic filling of both left and
right ventricle in the immediate as well as late postoperative period in the
majority of patients.
2. Presence of “annulus reversus” of mitral valve where
mitral lateral e¢ velocity was lower than medial e¢ velocity in all patients in this study before surgery.
3. Significant decrease of mitral medial e¢ velocity in early as well as late postoperative period. Following
pericardiectomy, the lateral e¢ velocity of the mitral valve exhibited
insignificant reduction in the immediate postoperative period and significant
reduction in the late postoperative period.
4. The identification of “annulus reversus” of the tricuspid
valve in all patients.
5. Exhibition of normalization of tricuspid
lateral/medial e¢ following pericardiectomy during follow-up.
6. Proportionately greater postoperative reduction in
tricuspid lateral e¢ velocity compared to mitral annulus values.
7. Demonstration of significant reduction in mitral and
tricuspid systolic annular velocity (lateral and medial) following
pericardiectomy in the postoperative period.
8. Exhibition of inspiratory decreases in peak
transmitral flow and inspiratory increase in transtricuspid flow in all
patients in the preoperative period. Following pericardiectomy, transmitral
early diastolic filling velocity continued to remain abnormal in 10 (18.5%)
patients upto 6 months. These symptomatic patients (n=10, 18.5%) continued to
have higher indexed IVC diameter and persistent atrial fibrillation (n=9) in
the postoperative period. Tissue Doppler imaging-derived mitral and tricuspid
annular velocities failed to predict the postoperative symptomatic status of
patients undergoing pericardiectomy (Tables 2-4); and
9. Preoperative atrial fibrillation was a predictor of
poor prognostic outcome following pericardiectomy.
Tissue Doppler imaging has made possible the
acquisition of myocardial wall velocities and offers incremental diagnostic
information to M-mode, 2D echo and transmitral flow Doppler for detecting
constrictive physiology with a reported sensitivity and specificity of 88.8%
and 94.8%, respectively [7,8,11-17]. Published data on the effect of
pericardiectomy on mitral and tricuspid annular velocities are limited because
of limited number of patients and restricted observations [12,16,17]. During
systole, the mitral annulus descends towards the apex, with no appreciable
motion of the apex in relation to the imaging transducer. Therefore, the
annular displacement reflects the extent of myocardial fiber shortening in the
longitudinal plane and has a strong linear correlation with global left
ventricular function [21]. Since the mechanoelastic properties of the
myocardium are preserved in CP, the longitudinal mitral annular velocities
remain normal or can be exaggerated as lateral expansion in constrictive
pericarditis is limited [12,13,17].
Previous investigators have evaluated the role of
tissue Doppler imaging in the diagnosis of CP in patients without diagnostic
respiratory variation of transmitral early diastolic filling velocity. They
concluded that in patients with preserved mitral e¢
velocity (>8 cm/s) and a low E/e¢ ratio (<8) with
high LV filling pressure, the recognition of “annulus reversus” should alert to
the diagnosis of CP [7-9,12-14,22]. Building on the above-mentioned
observations, we attempted to evaluate tissue Doppler imaging at mitral and
tricuspid annuli in patients undergoing pericardiectomy and identify the
relationship if any of the TDI-derived variables with patient’s symptomatic
status following surgery.
Early
diastolic mitral annulus velocity
We confirmed the presence of “annulus reversus” in
all patients with CP in the preoperative period. Following pericardiectomy, it
may be anticipated that the medial mitral annular velocity decreases and the
lateral annular velocity increases, resulting in normalization of
lateral/medial e¢ ratio. In this
study, while the latter was confirmed, both medial and lateral e¢ velocities were found to decrease after pericardiectomy and there was no
reversal (Table 4).
Veress et al. [17] had similar observations in their
study and described the following mechanisms for their observations:
Pericardiectomy removes constraint to lateral mitral annular expansion and
nullifies the exaggerated longitudinal mitral annular motion as well as the
translateral component of lateral e¢ velocity related
to increased medial excursion.
Early
diastolic tricuspid annulus velocity
The phenomenon of “annulus reversus” of the
tricuspid valve was observed in all patients in this study. There was reduced
lateral tricuspid annular velocity (e¢) in all patients
and normalization of the tricuspid lateral/medial e¢ ratio
following pericardiectomy during the follow-up period (Table 4).
Therefore, the above mentioned mechanisms operative at the mitral annulus may
as well be responsible for findings at the tricuspid annulus.
In this study, mild mitral and tricuspid
regurgitation was present in 8 (14.8%) patients. Both of them responded
favorably to pericardiectomy and postoperative conservative management. The
frequent association of CP with significant tricuspid regurgitation and
worsening of tricuspid regurgitation following pericardiectomy in a subset of
patients in the published literature are noteworthy [23].
Systolic
annulus velocity
Systolic annulus velocity (s¢) by
tissue Doppler imaging reflects the peak velocity of myocardial fiber
shortening in the longitudinal direction and provides a more sensitive
assessment of global left and right ventricular systolic function than 2D or
M-mode imaging. It was measured via an apical four chamber view at systole (s¢) with a 2-5 mm tissue Doppler sample volume placed at the septal corner
and at the mitral and tricuspid lateral annuli. s¢ has
been correlated with peak positive dP/dt and LVEF in patients with dilated
cardiomyopathy, and myocardial infarction [24,25]. There is little information
on mitral and tricuspid s¢ velocities in
patients with CP [17,26,27]. In this study, the correlation between mitral s¢ and LVEF was statistically insignificant (Table 4).
The mean s¢ velocity in all
patients in this study was lower both before and after pericardiectomy than
published normative values [28] and also lower, especially pre-pericardiectomy (Table
4). These observations are consistent with previous smaller studies
[17,26]. This finding contradicts the theoretical basis since velocity is
expected to increase with augmented stroke volume after pericardiectomy.
It is postulated that stroke volume in constrictive
pericarditis is closely coupled, in part via elastic recoil mechanisms. Thus,
in the pre-pericardiectomy setting, both longitudinal systolic and diastolic
motion of the annuli are exaggerated while following release of pericardial
constraint, both decrease in tandem. This hypothesis is supported by other
investigators demonstrating moderate to high correlation between annular s¢ and e¢ as well as s¢ and a’, especially before pericardiectomy when restorative forces may be
most operative [17].
There appeared to be proportionately greater
postoperative reduction in tricuspid lateral or right ventricle s¢ and e¢ compared to mitral
annulus values. Asymmetric distribution of the diseased pericardium
predominantly over the RV may well be responsible for the above observations.
However, the disproportionate reduction in tricuspid lateral s¢ and e¢ probably seems
also from postoperative RV dysfunction, which was moderate in 10 (18.5%)
patients.
Left ventricular ejection fraction did not change
despite the expected increase in stroke volume after pericardiectomy. It is
postulated that after pericardial resection, LV filling increases and other
elements of LV shortening including torsion are recruitable, contributing to better
cardiac output and compensating for abnormal longitudinal function [17].
Sengupta et al. [29] found higher net twist but no significant increase in
torsion post-pericardiectomy, a conclusion limited by small patient numbers and
early timing of the postoperative studies when restoration of function may have
been incomplete. To confirm this hypothesis, detailed analysis of myocardial
mechanics in a larger number of patients pre- and post-pericardiectomy will be
required.
Monitoring of intracardiac pressures during
pericardiectomy has been proposed to evaluate the result of decortications but
Viola [30] argued against the value of this assessment because further recovery
of myocardial failure may occur late after pericardiectomy. In this study, we
showed that there is a relationship between the degree of decrease in atrial
pressure after pericardiectomy and postoperative diastolic function. Secondly,
early abnormalities in diastolic filling pattern may improve in the late
follow-up; however, the long-term hemodynamic result may not be predicted by
the immediate postoperative Doppler echocardiographic findings.
It has been shown that diastolic filling
characteristics remain abnormal in a substantial number of patients with CP;
even after successful pericardiectomy, these abnormalities may resolve
gradually. Moreover, diastolic filling abnormalities after pericardiectomy
correlate well with clinical symptoms and tend to occur in patients who had
long- standing preoperative symptoms [1-4,6,31].
In our study group, 10 (18.5%) patients continued to
have NYHA Class II symptoms late postoperatively. However, none of them had
raised jugular venous pulsation, hepatomegaly or ascites. These patients
exhibited higher RA pressure measured via central venous catheter, increased
indexed IVC diameter, higher LVID and persistently abnormal transmitral early
diastolic filling velocity in the postoperative period, as compared to the
asymptomatic patients (Tables 2-4). During surgery, these patients had
extensive pericardial calcification over the anterior and inferior surfaces of
the right and left ventricle. However, total pericardiectomy including removal
of the calcified pericardium overlying the anterolateral and diaphragmatic
surface of the right ventricle was achieved in all patients of the study group.
These patients in the immediate postoperative period required higher inotropic
support because of low cardiac output. We believe that subjecting the newly
liberated right, and perhaps left ventricle to even moderately elevated filling
pressure led to increased wall stress and deteriorating cardiac function.
It is pertinent to state that there were no
differences of TDI-derived systolic and diastolic annular velocities of the
mitral and tricuspid valves between symptomatic and asymptomatic patients in
the preoperative period. Therefore, the TDI-derived mitral and tricuspid
annular velocities failed to predict the symptomatic status of patients
undergoing pericardiectomy. It is also worthwhile to mention that 9 out of 10
patients who were symptomatic in the postoperative period continued to remain
in atrial fibrillation. Therefore, the presence of atrial fibrillation in the
preoperative period may be a predictor of poor prognostic outcome following
pericardiectomy. The utility of tissue Doppler imaging in identifying residual
constrictive pericarditis requires further investigation on a large cohort of
patients correlating the clinical outcomes.
STUDY
LIMITATIONS
Majority of patients in this study underwent total
pericardiectomy via median sternotomy. Hence, the tissue Doppler imaging
variables could not be compared with anterolateral thoracotomy approach. The
small number of postoperative symptomatic patients in this study is an
additional limitation.
Secondly, heart performs complex rotational and
translational movement inside the chest, thus distorting the measurements of
myocardial velocities. In this study, we only recorded tissue Doppler imaging
of longitudinal axis motion in the 4-chamber view. Due to the local tethering
effect, analysis of multiple annular regions could have provided additional
helpful data. Studies are underway to analyze radial and circumferential
function for a better understanding of the mechanics of the unique annular
motion in constrictive pericarditis and effects of pericardiectomy [32].
CONCLUSION
This study
demonstrates that patients with congestive heart failure and normal LVEF,
preserved or increased mitral medial e¢ velocity with “annulus
reversus” is diagnostic of constrictive pericarditis. This characteristic
pattern of annular velocities return to normal after pericardiectomy. The
extent of postoperative changes is maximal in the immediate postoperative
period. Tissue Doppler imaging-derived mitral and tricuspid annular velocities
cannot predict the postoperative outcome of patients undergoing
pericardiectomy. Tissue Doppler imaging is a useful investigative modality for
diagnosis of constrictive pericarditis and not a useful indicator of
postoperative evaluation.
1. Ling LH,
Oh JK, Schaff HV, Danielson GK, Mahoney DW, et al. (1999) Constrictive
pericarditis in the modern era: Evolving clinical spectrum and impact on
outcome after pericardiectomy. Circulation 100: 1380-1386.
2. De
Valeria PA, Baumgartner WA, Cásale AS, Greene PS, Cameron DE, et al. (1991)
Current indications, risks and outcome after pericardiectomy. Ann Thorac Surg
52: 219-224.
3. Chowdhury
UK, Subramaniam G, Kumar AS, Airan B, Singh R, et al. (2006) Pericardiectomy for
constrictive pericarditis: Clinical, echocardiographic and hemodynamic
evaluation of two surgical techniques. Ann Thorac Surg 81: 522-530.
4. Chowdhury
UK, Seth S, Reddy SM (2008) Pericardiectomy for chronic constrictive
pericarditis via left anterolateral thoracotomy. Operative Techniques in
Thoracic and Cardiovascular Surgery: A Comparative Atlas 13: 14-25.
5. Chowdhury
UK, Narang R, Malhotra P, Choudhury M, Choudhury A (2016) Indications, timing
and techniques of radical pericardiectomy via modified left anterolateral
thoracotomy (UKC’s modification) and total pericardiectomy via median
sternotomy (Holman and Willett) without cardiopulmonary bypass. J Pract
Cardiovasc Sci 2: 17-27.
6. McCaughan
BC, Schaff HV, Piehler JM, Danielson GK, Orszulak TA, et al. (1985) Early and
late results of pericardiectomy for constrictive pericarditis. J Thorac
Cardiovasc Surg 89: 340-350.
7. Garcia
MJ, Rodriguez L, Ares M, Griffin BP, Thomas JD, et al. (1996) Differentiation
of constrictive pericarditis from restrictive cardiomyopathy: Assessment of
left ventricular diastolic velocities in longitudinal axis by Doppler tissue
imaging. J Am Coll Cardiol 27: 108-114.
8. Rajagopalan
N, Garcia MJ, Rodriguez L, Murray RD, Apperson-Hansen C, et al. (2001)
Comparison of new Doppler echocardiographic methods to differentiate
constrictive pericardial heart disease and restrictive cardiomyopathy. Am J
Cardiol 87: 86-94.
9. Ha JW,
Oh JK, Ling LH, Nishimura RA, Seward JB, et al. (2001) Annulus paradoxus:
Transmitral flow velocity to mitral annular velocity ratio is inversely
proportional to pulmonary capillary wedge pressure in patients with
constrictive pericarditis. Circulation 104: 976-978.
10. Miyatake
K, Yamagishi M, Tanaka N (1995) New method for evaluating left ventricular wall
motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J
Am Coll Cardiol 25: 717-724.
11. Oh JK,
Hatle LK, Seward JB, Danielson GK, Schaff HV, et al. (1994) Diagnostic role of
Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol 23:
154-162.
12. Kim JS,
Ha JW, Im E, Park S, Choi EY, et al. (2009) Effects of pericardiectomy on early
diastolic mitral annular velocity in patients with constrictive pericarditis.
Int J Cardiol 133: 18-22.
13. Reuss
CS, Wilansky SM, Lester SJ, Lusk JL, Grill DE, et al. (2009) Using mitral
'annulus reversus' to diagnose constrictive pericarditis. Eur J Echocardiogr
10: 372-375.
14. Sengupta
PP, Mohan JC, Mehta V, Arora R, Pandian NG, et al. (2005) Accuracy and pitfalls
of early diastolic motion of the mitral annulus for diagnosing constrictive
pericarditis by tissue Doppler imaging. Am J Cardiol 93: 886-890.
15. Mor-Avi
V, Lang RM, Badano LP, Belohlavek M, Cardim NM, et al. (2011) Current and
evolving echocardiographic techniques for the quantitative evaluation of
cardiac mechanics: ASE/EAE consensus statement on methodology and indications
endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12:
167-205.
16. Mor-Avi
V, Lang RM, Badano LP (2011) Current and evolving echocardiographic techniques
for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus
statement on methodology and indications endorsed by the Japanese Society of
Echocardiography. Eur J Echocardiogr 12: 167-205.
17. Sohn DW,
Kim YJ, Kim HS, Kim KB, Park YB, et al. (2004) Unique features of early
diastolic mitral annulus velocity in constrictive pericarditis. J Am Soc
Echocardiogr 17: 222-226.
18. Veress
G, Ling LH, Kim KHJ, Dal-Bianco JP, Schaff HV, et al. (2011) Mitral and
tricuspid annular velocities before and after pericardiectomy in patients with
constrictive pericarditis. Circ Cardiovasc Imaging 110: 959-619.
19. Quinones
MA, Pickering E, Alexander JK (1978) Percentage of shortening of the echocardiographic
left ventricular dimension. Its use in determining ejection fraction and stroke
volume. Chest 74: 59-65.
20. Jiamsripong
P, Honda T, Reuss CS, Hurst RT, Chaliki HP, et al. (2008) Three methods for
evaluation of left atrial volume. Eur J Echocardiogr 9: 351-355.
21. Cheitlin
MD, Armstrong WF, Aurigemma GP (2003) ACC/AHA/ASE guideline update for the
clinical application of echocardiography: Summary article. A report of the
American College of Cardiology/American Heart Association Task Force on Practical
Guidelines. Circulation 108: 1146-1162.
22. Zaky A,
Grabhorn L, Feigenbaum H (1967) Movement of the mitral ring: A study in
ultrasound cardiography. Cardiovasc Res 1: 121-131.
23. Nagueh
SF, Middleton KJ, Kopelen HA, Zoghbi WA (1997) Doppler tissue imaging: A
non-invasive technique for evaluation of left ventricular relaxation and
estimation of filling pressures. J Am Coll Cardiol 30: 1527-1533.
24. Johnson
TL, Baughman WB, Josephson RA (1993) Worsening tricuspid regurgitation
following pericardiectomy for constrictive pericarditis. Chest 104: 79-81.
25. Yamahada
H, Oki T, Tabata T (1998) Assessment of left ventricular systolic wall motion
velocity with pulsed tissue Doppler imaging: Comparison with peak dP/dt of the
left ventricular pressure curve. J Am Soc Echocardiogr 11: 442-449.
26. Alam M,
Wardell J, Andersson E, Samad BA (2000) Effects of first myocardial infarction
on left ventricular systolic and diastolic function with the use of mitral
annular velocity determined by pulsed wave Doppler tissue imaging. J Am Soc Echocardiogr
13: 343-352.
27. Choi EY,
Ha JW, Kim JM, Ahn JA, Seo HS, et al. (2007) Incremental value of combining
systolic mitral annular velocity and time difference between mitral inflow and
diastolic mitral annular velocity to early diastolic annular velocity for
differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am
Soc Echocardiogr 20: 738-743.
28. Homsi M,
Mahenthiran J, Vaz D, Sawada SG (2007) Reduced right ventricular systolic
function in constrictive pericarditis indicates myocardial involvement and
persistent right ventricular dysfunction and symptoms after pericardiectomy. J
Am Soc Echocardiogr 20: 1417.e1-1417.e7.
29. Chahal
NS, Lim TK, Jain P, Chambers JC, Kooner JS, et al. (2010) Normative reference
values for the tissue Doppler imaging parameters of left ventricular function:
A population-based study. Eur J Echocardiogr 11: 51-56.
30. Sengupta
PP, Krishnamoorthy VK, Abhayaratna WP, Korinek J, Belohlavek M, et al. (2008)
Disparate patterns of left ventricular mechanics differentiate constrictive
pericarditis from restrictive cardiomyopathy. JACC Cardiovasc Imaging 1: 29-38.
31. Viola AR
(1973) The influence of pericardiectomy on the hemodynamics of chronic
constrictive pericarditis. Circulation 48: 1038-1042.
32. Senni M,
Redfield MM, Ling LH, Danielson GK, Tajik AJ, et al. (1999) Left ventricular
systolic and diastolic function after pericardiectomy in patients with
constrictive pericarditis: Doppler echocardiographic findings and correlation
with clinical status. J Am Coll Cardiol 33: 1182-1188.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Alcoholism Clinical Research
- Oncology Clinics and Research (ISSN: 2643-055X)