694
Views & Citations10
Likes & Shares
Excellent progress
has been made in the past two decades in understanding peripartum
cardiomyopathy (PPCM). A strategy is outlined for steps that can be taken
before, during and after pregnancy to improve outcomes for subsequent
pregnancies following recovery from a diagnosis of heart failure from
peripartum cardiomyopathy. This strategy has helped to save the lives of
mothers and children. Treatment and monitoring strategies can also be used to
reach “full recovery” with the first diagnosis of PPCM.
Keywords: Heart failure, Pregnancy, Cardiomyopathy
INTRODUCTION
Excellent progress has been made in the past two decades in understanding peripartum cardiomyopathy (PPCM) [1-5]. The pathophysiology and treatment of PPCM have become much clearer. In this process I outline an effective path in guiding PPCM mothers in their quandaries about the risks of a subsequent pregnancy. Herein, I present important considerations relative to the safety and advisability of any future pregnancies:
What can a peripartum cardiomyopathy (PPCM) subject do to reduce the risks of relapse of heart failure in a subsequent pregnancy?
Before the post-PPCM pregnancy
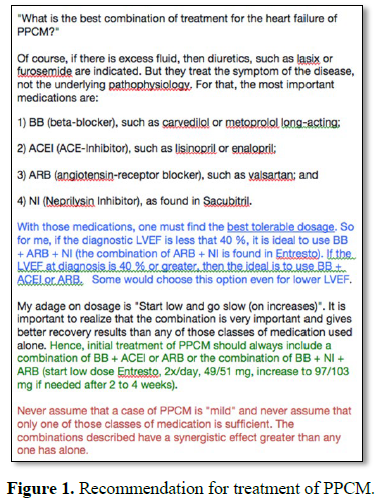
1) Adequate treatment of first episode to help reach left ventricular ejection fraction (LVEF) of 55% (anything higher considered a “variant of normal”) [6] (Figure 1).
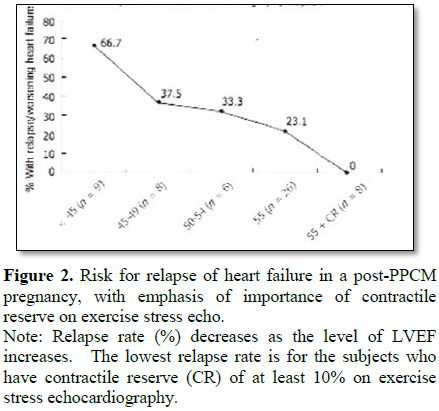
2) Be sure that “contractile reserve” is adequate; defined as increase of LVEF by at least 10-15% from resting heart rate to target exercise heart rate on exercise stress echocardiography (example: from LVEF 55% to 63%) [5-9] (Figure 2).
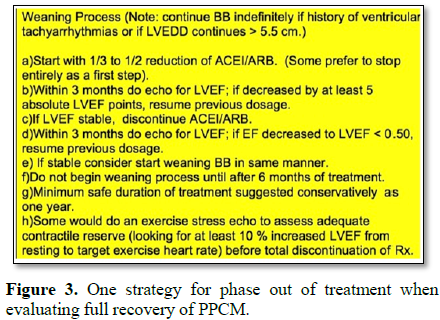
3) Maintain normal heart function (LVEF 55%) after phase-out of medication that would not be safe during conception/pregnancy; such as Angiotensin-Converting Enzyme Inhibitor (ACEI) or angiotensin hormone receptor blocker (ARB) [10] (Figure 3).
4) “Full recovery” confers lower risk, and means
a) Return of LVEF to ≥ 55%;
b) Normal contractile reserve on exercise stress echocardiography;
c) No deterioration of LVEF when wean off ARB/ACEI Rx;
d) No diastolic dysfunction; if that exists more Rx indicated;
e) No late Gadolinium enhancement (LGE) on cardiac MRI (CMR) [11];
f) Size of left ventricle <6 cm or <3.5 cm/M2 BSA.
During the post-PPCM pregnancy
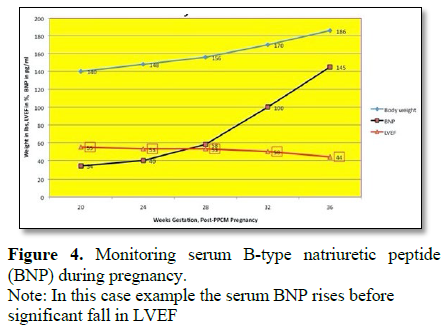
1) Establish base-line serum B-type Natriuretic Peptide (BNP) and monitor serum BNP level each trimester. Look for levels that are rising and/or above “cut-off” level for that particular lab’s test. The NT-ProBNP test is recommended because it appears to be the least affected by pregnancy alone (Figure 4).
2) Monitor LVEF by echocardiography each trimester and more often if serum BNP is rising and/or above “cut-off” value.
3) Rising serum BNP above “cut-off” is an indication to consider starting a treatment because the rise in serum BNP comes hours to days to weeks before any fall in LVEF.
4) Monitor the “self-test” monthly for recognition of signs and symptoms of heart failure during pregnancy [12]. The “self-test” consists of 6 clinical signs/symptoms:
a. Orthopnea (difficulty breathing when lying flat): (a) None 0 points; (b) Need to elevate head 1 point; (c) Need to elevate 45° or more 2 points.
b. Dyspnea (shortness of breath on exertion): (a) None 0 points; (b) Climbing 8 or more steps 1 point; (c) Walking on level 2 points.
c. Unexplained cough: (a) None 0 points; (b) At night 1 point; (c) Day and night 2 points.
d. Swelling (pitting edema) lower extremities: (a) None 0 points; (b) Below knee 1 point; (c) Above and below knee 2 points.
e. Excessive weight gain during last month of pregnancy: (a) Under 2 pounds per week 0 points; (b) 2 to 4 pounds per week 1 point; (c) Over 4 pounds per week 2 points.
f. Palpitations (sensation of irregular heartbeats): (a) None 0 points; (b) When lying down at night 1 point; (c) Day and night, any position 2 points.
ACTION: 5 or more points=see cardiologist re: plasma BNP and echocardiogram.
5) Watch for further developments of this research tool (angiogenic imbalance) [5,13-15]:
a. Serum soluble FLT1 >100 pg/ml
b. Ratio of serum sFLT1 to Placenta Growth Factor (PlGF)>45 @ 24 weeks gestation. This may be predictive of trouble at 32 weeks gestation. What applies to preeclampsia may also apply to PPCM [15].
6) Development of gestational hypertension or preeclampsia during pregnancy identifies higher risk for relapse of heart failure in a post-PPCM pregnancy [1,3,5,14] because:
a. One-third to one-half of PPCM mothers also has either preeclampsia or gestational hypertension.
b. Pathophysiology of these two conditions appears to have many similarities to the pathophysiology of PPCM, particularly the angiogenic imbalance resulting from alteration of normal patterns of placental production of sFLT-1 and/or PlGF.
c. These two conditions alert the medical team to the need for even closer monitoring of the pregnancy; and the need to initiate effective treatment for the prevention or moderation of relapse of heart failure or subsequent deterioration of LVEF.
7) Effective treatment [16,17] is available if there is pending relapse during pregnancy; this treatment consists of:
a. Use of beta-blockers (BB), which are safe to use during pregnancy. Some prefer to keep BB in the picture from the time of beginning the subsequent pregnancy.
b. Hydralazine, in tolerable dosages, can take the place of ACEIARB, which are not safe to take during pregnancy. If the hydralazine gives unacceptable increase of heart rate, this side-effect may be off-set by the use of nitrates, which are also safe to take during pregnancy. Hydralazine is more a “second-class” medication, with more potential side-effects; so closer monitoring is necessary.
8) Completion of pregnancy, with safe maturity of the infant, is the best measure to eliminate any further risk for relapse of heart failure. In completing the pregnancy, there are important considerations:
a. If there has been any suggestion of beginning relapse, use of pitocin stimulation of labor should be avoided because it increases stress on the left ventricle [18]. This is reinforced by the author’s case file which includes 5 additional PPCM mothers experiencing worsening heart failure after/during pitocin drip induction/stimulation of labor.
b. If there is no suggestion of relapse, then earlier onset of labor may be encouraged by induction methods of stripping the membranes through the beginning cervical dilatation; and pitocin stimulation of labor contractions appears to be safe when no pending/developing relapse.
c. Consideration for elective Caesarean section with maturity of baby is a safe alternative when quality anesthesia services are available.
After the post-PPCM pregnancy
1) If there has been no evidence for relapse of heart failure or trend towards relapse:
a. It is important to continue to monitor for evidence of relapse up to approximately 5-6 months postpartum.
b. An echocardiogram should be done 6 months postpartum in order to assure that no deterioration has occurred.
2) If there is evidence for relapse or trend towards relapse, treatment must be continued and intensified.
a. At this point, it is better to replace the hydralazine with ACEI or ARB;
b. However, if breastfeeding, the only ACEI that is safe to use is enalopril; and no ARB is deemed safe to use while breastfeeding.
c. It has not yet been determined if lactate hormone inhibition with use of bromocriptine or cabergoline is safe and effective [4,19-21].
CONCLUSION
From extensive experience with hundreds of PPCM subjects, I have seen this strategy work. It has helped to save the lives of mothers and children. Please consider some or all of these points in the management and treatment of those who experience or plan for subsequent pregnancy after having a diagnosis of PPCM.
1. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, et al. (2015) IPAC investigators. Clinical outcomes for peripartum cardiomyopathy in North America: Results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy. J Am Coll Cardiol 66: 905-914.
2. Warraich RS, Sliwa K, Damasceno A, Carraway R, Sundrom B, et al. (2005) Impact of pregnancy-related heart failure on humoral immunity: Clinical relevance of G3-subclass immunoglobulins in peripartum cardiomyopathy. Am Heart J 150: 263-269.
3. Elkayam U (2011) Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis and management. J Am Coll Cardiol 58: 659-670.
4. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, et al. (2013) management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 108: 366.
5. Fett JD (2014) Peripartum cardiomyopathy: A puzzle closer to solution. World J Cardiol 6: 81-114.
6. Fett JD, Shah TP, McNamara DM (2015) Why do some recovered peripartum cardiomyopathy mothers experience heart failure with a subsequent pregnancy? Cardiovasc Med 17: 354.
7. Fett JD (2005) Outcomes for subsequent pregnancies following pregnancy of diagnosis of peripartum cardiomyopathy. Circulation 112: 593-2808.
8. Fett JD, Sannon H, Thélisma E, Sprunger T, Venkita S (2009) Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynecol Obstet 104: 125-127.
9. Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, et al. (2010) Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol 154: 27-31.
10. Fett JD, Fristoe KL, Welsh SN (2010) Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet 109: 34-36.
11. Schelbert EB, Elkayam U, Cooper LT, Givertz MM, Fett JD, et al. (2017) Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc 6: e005472.
12. Fett JD (2011) Validation of a self-test for early diagnosis of heart failure in peripartum cardiomyopathy. Crit Pathw Cardiol 10: 44-45.
13. Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, et al. (2016) Relaxin-2 and soluble FLT-1 levels in peripartum cardiomyopathy: Results of the multicenter IPAC study. JACC Heart Fail 4: 380-388.
14. Patten IS, Rana S, Shahul S, Rowe GC, Jang C, et al. (2012) Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333-338.
15. Sabria E, Lequerica-Fernandez P, Ganuza PL, Angeles EE, Escudero AI, et al. (2018) Use of the Flt-1/PlGF ratio to rule out preeclampsia requiring delivery in women with suspected disease. Is the evidence reproducible? Clin Chem Lab Med 58: 303-311.
16. Fuster V (2009) American Heart Association. The AHA Guidelines and Scientific Statements Handbook. Wiley-Blackwell. Oxford, UK.
17. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, et al. (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Eur Heart J 33: 1787-1847.
18. Shimazake M, Arai T, Matsuda Y (2008).Case of peripartum cardiomyopathy developed after an emergency cesarean section - A case report. Masui 57: 213-215
19. Hopp L, Weisse AB, Iffy L (1996) Acute myocardial infarction in a healthy mother using bromocriptine for milk suppression. Can J Cardiol 12: 415-418.
20. Ruch A, Duhring JL (1989) Postpartum myocardial infarction in a patient receiving bromocriptine. Obstet Gynecol 74: 448-451.
21. Bernard N, Jantzem H, Becker M (2015) Severe adverse effects of bromocriptine in lactation inhibition: A pharmacovigilance survey. BLOG 122: 1244-1251.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Alcoholism Clinical Research
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Spine Diseases
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)