923
Views & Citations10
Likes & Shares
During
life, the brain undergoes several structural and functional changes. The nature
of such changes is of paramount importance to define the conditions of
normality and to distinguish physiological changes from those resulting from
neuropathological processes. The purpose of this study was to elucidate the
major functional changes associated with age and with specific genotypes. We
found that age produces non-linear regional changes in brain activity and
disruptions between cortical networks affecting the alpha2 band in
particular. Increased alpha1 oscillations in parietal lobe and decreased
alpha2 oscillations in occipital lobe, together with functional
disruptions in parieto-frontal connections likely represent neurophysiological
markers of normal aging. On the other hand, we found that the APOE-4
allele has an influence on brain activity even in non-demented healthy
subjects. The CYP gene family may also affect brain function, and SNPs
of the AGT gene associated with arterial hypertension and
cerebrovascular pathology influence brain activity in patients with vascular
dementia.
INTRODUCTION
The human
brain is a dynamic system that shows both structural and functional changes
from the fetus to the elderly. During this process, different brain regions and
systems mature and degenerate along different timelines, finally resulting in
the aging brain. The aging brain is characterized by (i) thinning of the
cortex, (ii) loss of neural circuits and brain plasticity, (iii) alterations in
gene expression and (iv) deficit in synthesis and transport of
neurotransmitters [1-3]. All these signs associated with
physiological aging are strongly influenced by the presence of risk variants in
key genes associated with body homeostasis (e.g. APOE, AGT), drug
metabolism (phase I (CYPs) and phase II reactions (UGTs, NATs))
and drug transporters (ABCs, SLCs). Similarly, chronic treatment
with drugs affecting the central nervous system (CNS) and consumption of drugs
of abuse and toxic substances induce dramatic changes in the brain. In
addition, epigenetics affects life span and longevity. Epigenetic alterations
are present in different tissues throughout the aging process and in
neurodegenerative disorders, such as Alzheimer's disease (AD). AD-related genes
exhibit epigenetic changes, indicating that epigenetics might exert a
pathogenic role in dementia [4]. The different forms of dementia
pose several challenges to our society and the scientific community: (i) they
represent an epidemiological problem, and a socioeconomic, psychological and
family burden; (ii) most of them have an obscure/complex pathogenesis; (iii)
their diagnosis is not easy and lacks specific biomarkers; and (iv) their
treatment is difficult and inefficient [5].
Neuroimaging
studies have provided substantial evidence about structural changes but the
functional alterations associated with age or genotype remain largely unclear.
The quantitative analysis of electroencephalography (EEG) is a low-cost
approach to study brain function, allowing the visualization of neural activity
with a high time resolution. Previous EEG studies have revealed a slowing of
EEG pattern in normal elderly subjects [6,7].
EEG is useful not only to discriminate patients from healthy subjects,
but also for the prediction of treatment outcome in various brain diseases,
yielding information about tailored therapy approaches for an individual [8].
AD patients treated with citicoline show more alpha (occipital
electrodes) and theta (left side electrodes), accompanied by less delta
activity in the left temporal lobe. Furthermore, significant differences with
respect to placebo have been observed for theta activity in several
fronto-parieto-temporal electrodes in the left hemisphere [9]. Cerebrolysin
induces reductions in delta and theta activities in post acute
moderate-severe traumatic brain injury (TBI) patients, showing good correlation
with improvement of attention and working memory [10]. A decrease of theta activity over all cortical regions,
increase of beta activity, and some restoration of the occipital alpha
rhythm have been seen in Rett syndrome
patients treated with cerebrolysin [11]; however, analysis of brain
activity merely according to anatomically separated responses is insufficient
to understand the complexity of functional changes in the brain. Functional
connectivity is commonly assessed during performance of a cognitive task.
Particular attention has been given to the inherent functional organization of
brain networks in resting state. The brain resting state is an energetically
costly condition characterized by a rich neural activity and long-range
interneuron connections in specific brain circuits (e.g. DMN, default mode
network). It has been suggested that resting-state networks may reflect an
intrinsic property of brain functional organization that serves to stabilize
brain ensembles, consolidate the past, and prepare us for the future [12, 13].
To visualize resting-state synchronization across frequency bands in
large-scale functional networks, two lagged functional connectivity measures
(lagged coherence and lagged phase synchrony), implemented in the eLORETA
statistical package, have been proposed. These connectivity indices are
resistant to non-physiological artifacts, in particular low spatial resolution
and volume conduction [14].
AGE-RELATED CHANGES IN BRAIN
ACTIVITY
Although age is the main source of physiological changes, little is
known about the functional organization of neural networks and its connection
with aging, neurodegenerative disorders and cerebro-vascular pathology. With
the aim of identifying the main age-related functional changes, we investigated
the brain activity of healthy subjects between 19 and 91 years of age. One
hundred eighty-one healthy subjects that visited EuroEspes Biomedical Research
Center for a clinical check-up were divided into three groups according to
their age: 28 young (A group; age range: 19-35 years, mean: 28.45 ± 5.03),
92 middle-aged (B group; age range: 36-59 years, mean: 48.5 ±
6.81), and 61 older (C group; age range: 60-91 years, mean: 67.50 ±
6.82). No participants had any cognitive disturbance or history of neurological
or psychiatric disorders. They were not taking any medication that might affect
CNS at the time of the study, and underwent brain MRI screening to exclude any
organic lesions.
EEG recordings were obtained in relaxed wakefulness with eyes closed by
using 19 scalp electrodes located according to the international 10-20 system.
The EEG activity was acquired using a linked ears reference, sampled at 500 Hz,
and filtered offline between 1 and 30 Hz. Analysis was circumscribed to the
resting, awake, eyes-closed state. For each subject, 20 non-overlapping, 2s
artifact-free segments were randomly selected. In particular, we carefully
avoided epochs containing ocular movements, muscle or cardiac contamination,
drowsiness signs (i.e. emergence of slow wave activity with suppression of alpha
rhythm), and even small baseline shifts so that reliable estimates of brain
function in the awake resting-state could be obtained. Further analyses were
performed using the eLORETA software. Functional images of spectral density
were computed for six frequency bands: delta (1.5-4 Hz), theta
(4-8 Hz), alpha1 (8-10 Hz), alpha2 (10-13 Hz), beta1
(13-21 Hz) and beta2 (21-30 Hz).
We performed a regression analysis including all participants. In
addition, we searched for significant differences in source localization and
functional connectivity between the three age groups.
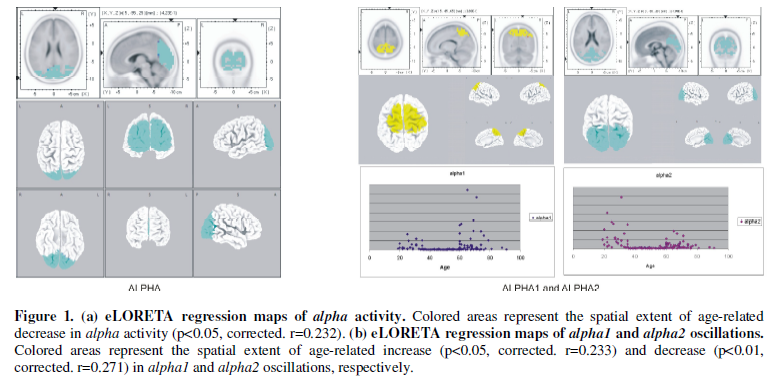
Regression analysis revealed a significant age-related decrease in the alpha
activity (8-13 Hz) in posterior regions (Figure
1(a)). Dividing alpha activity in alpha1 (8-10 Hz) and alpha2
(10-13 Hz) oscillations, we found a significant increase in alpha1
oscillations in parietal regions (best match in Brodmann area 7), and a
significant decrease in alpha2 oscillations in occipital cortex (best
match in Brodmann area 18) according to age (Figure 1(b)).
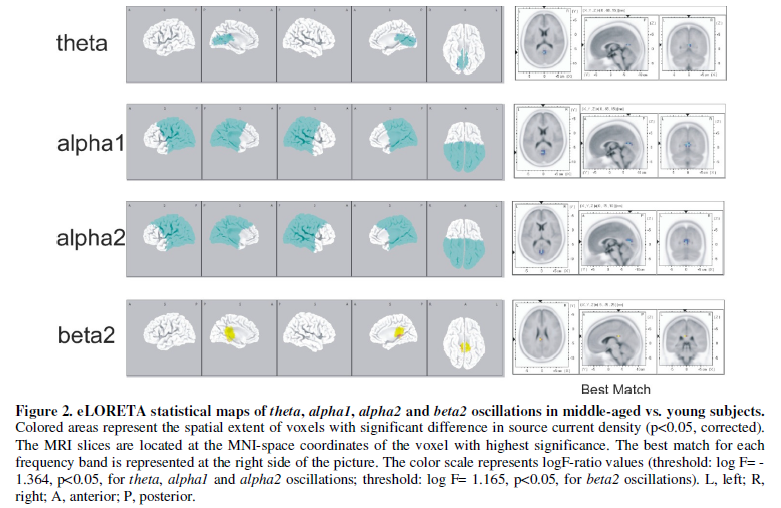
The middle-aged subjects (B group) exhibited significantly fewer theta,
alpha1 and alpha2 oscillations, and more beta2 activity
than the young subjects (A group), with the limbic lobe (posterior cingulate)
showing the highest significance for theta, alpha1 and beta2
activities, and the cuneus for alpha2 band (Figure 2).
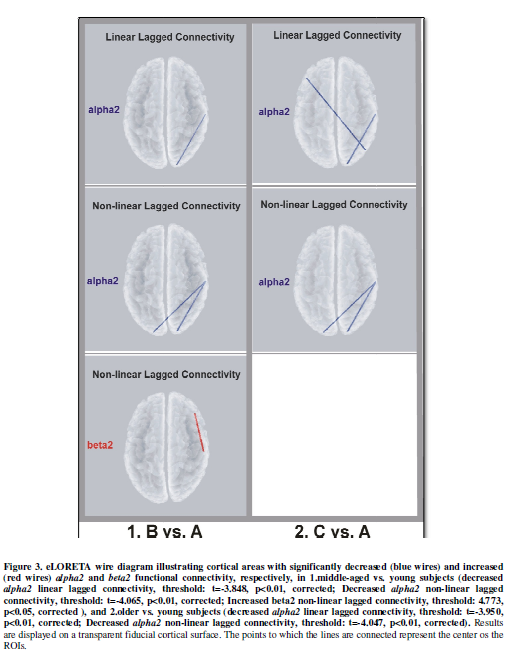
The connectivity pattern was characterized by reduced alpha2
lagged linear connectivity (LLC) between occipital and temporal cortex (O2-T8)
in the right hemisphere, and reduced alpha2 lagged non-linear
connectivity (LNL) between bilateral occipital and right temporal cortex (O1-T8
and O2-T8) in middle-aged subjects. In addition, there was increased LNC in the
beta2 band, involving right centro-frontal connections (C4-F8) (Figure 3).
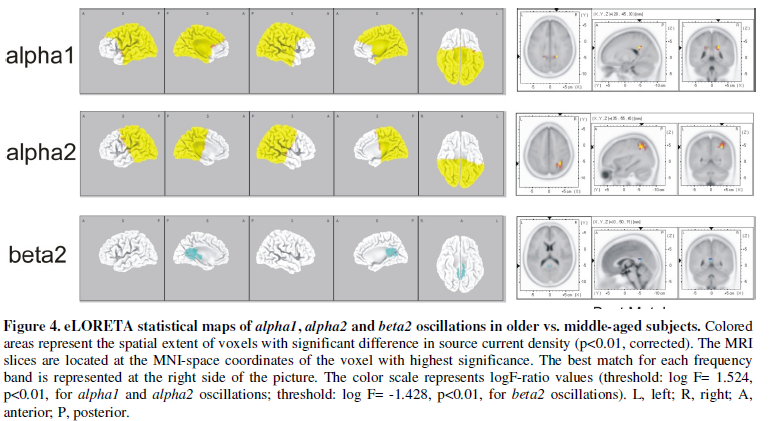
The older subjects (C group) exhibited significantly more alpha1
and alpha2 oscillations and fewer beta2 oscillations than the
middle-aged subjects (B group), with the right parietal lobe showing the
highest significance for alpha1 and alpha2 activities, and the
limbic lobe for beta2 oscillations (Figure
4). We found no significant difference in functional connectivity. However,
a decrease in beta1 connectivity in centro-parietal regions (Cz-P7)
nearly reached statistical significance (p<0.07, corrected).
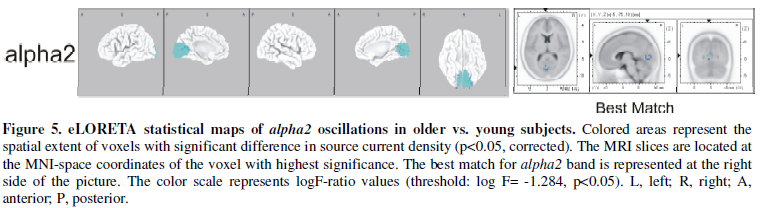
The older subjects (C group) exhibited a significant reduction in alpha2
oscillations in the occipital cortex compared with the young subjects (A group)
(Figure 5). In comparison with young
subjects, older subjects exhibited reduced alpha2 LLC in
occipito-temporal (O2-T8) and interhemispheric parieto-frontal circuits
(P4-F7). In addition, reductions in alpha2 LNC were seen between
bilateral occipital and right temporal cortex (O1-T8 and O2-T8). (Figure 3).
Alpha activity (8-13 Hz) suffers a significant
age-related decrease in posterior regions, notably in the occipital lobe (Figure 1). Decreased magnitude of
posterior alpha source has been seen by other authors [6,7] and
it may be associated with early changes in the functioning of the cholinergic
basal forebrain system. Since the main alpha generators under resting
conditions are the thalamus together with the cuneus and precuneus [15], our
data may reveal an age-related alteration of functional integrity of
thalamo-cortical circuits. Decreased resting alpha rhythms had been
induced by experimental impairment of cholinergic pathways stemming from the
basal forebrain [16] and patients that suffer an
evident impairment of cholinergic basal forebrain, such as cases with AD,
exhibit low posterior alpha power in EEG studies [17-21].
Following the methodology used by other authors, we divided the alpha
activity into its alpha1 (8-10 Hz) and alpha2 (10-13 Hz)
components. Interestingly, unlike Babiloni who saw less magnitude in both alpha1
and alpha2 sources [17], we found a significant
age-related increase in alpha1 oscillations in parietal regions,
especially in the left Brodmann area (BA) 7 (Figure 1). Given that the alpha oscillations are inverse
related to brain activity [22] and greater alpha power is
indicative of less cortical activity in broad underlying regions [23], our
data suggest that age induces a significant decrease in cortical activity
affecting particular areas of the parietal lobe. Of note, these areas are part
of the default mode network (DMN), a brain circuit typically active during
rest, whose correlation with EEG alpha activity is well known [24,25].
Therefore, older subjects show an altered DMN due to a decreased parietal
activity. Since the integrity of parietal cortex is fundamental to maintain
good cognitive performance and sense-spatial perception, parietal impairment
may be implicated in the decline of brain function traditionally associated
with old age. Several studies have demonstrated the relationship between
cognitive decline in both normal and demented elderly with impairment of the
parietal lobe and/or the DMN [26-29]. However, since alpha1
activity responds selectively to attentional demands [30], an
increase at rest may be necessary to maintain an adequate level of attention
and alertness in healthy non-demented subjects and it may be a cosubstantial
sign of healthy aging. Interestingly, the loss of parietal alpha1 activity
has been related to pathological processes in AD [18]. The slowing and shift to
anterior regions of alpha activity associated with age (less alpha2 in
occipital lobe and more alpha1 in parietal lobe) are consistent with
those of classical EEG studies that found slowing of the alpha peak
during physiological aging [30,31].
Brain activity in young vs. middle-aged
subjects
We found several significant differences in both cortical oscillations
and functional connectivity. Compared with the young, middle-aged subjects
showed: (i) significant reduction in theta oscillations and increase in beta2
oscillations in the limbic lobe; and (ii) significant decrease of alpha1
and alpha2 oscillations in frontal, temporal, parietal, limbic and
occipital lobes (Figure 2). In
addition, we found both a significant increase in the beta2 connectivity
in right centro-frontal connections and a decrease in the alpha2
connectivity between right temporal cortex and occipital lobe (Figure 3). These findings suggest that
middle-aged subjects have an energetically more costly resting state
characterized by higher activity in frontal, temporal, parietal, occipital (alpha
desynchronization), and limbic lobes (more beta2 together with less theta
and alpha oscillations). On the other hand, both age groups may differ
in their attention and cognition processes, as suggested by changes in cortical
activity and the functional connectivity of long-range networks. In humans,
previous studies have reported significant correlations between EEG data and
simultaneously recorded BOLD signal fluctuations within specific resting
networks. In particular, at rest, regions of the DMN associated with internal
processing (e.g. PCC) increase their beta oscillations, and the resting
state dorsal attention network, involved in attention and related cognitive
processes, shows a decrease in its alpha oscillations [32].
Both functional signatures are present in middle-aged compared to young
subjects. The observed changes in brain activity may originally be caused by
the imbalance induced by an early loss of Temporo-Occipital (T-O) connectivity.
In the middle-aged subjects, the decreased alpha2 connectivity involves
changes in the functional organization of long-range cortical networks
presumably affecting sensorial processing, with disconnections between primary
visual areas and right associative visual cortex. The loss of cortical
connectivity in these subjects probably causes an increased level of cortical
activity, namely decreases in alpha1 and alpha2. In fact, since
the alpha oscillations play a general inhibitory role on cognitive and
sensory processing [33,34], the decrease in alpha
oscillations at rest may involve a lower stimulus detection threshold (sensory
processing) and/or stronger cognitive processing at rest (e.g. abortive
orienting reactions or loadings of working memory loops that occur
spontaneously during conscious rest). Furthermore, good perception performance
is related to low alpha power at rest [35].
This may be interpreted in terms of cortical inhibition and excitation
previous to task performance [33]. According to this
interpretation, perception performance is enhanced if the cortex is already
activated, whereas memory performance is enhanced if the cortex is deactivated
at rest before a task is performed (several studies have shown that high
resting alpha power is positively associated with task performance [36,37,38]).
This interpretation is plausible if, for sensorial discrimination, a high level
of cortical excitation is helpful to analyze a sensorial input. For memory
performance (and other cognitive processes) initial activation of the cortex
may be detrimental because it may interfere with the high selectivity that is
required to access a memory trace [33]. In a scenario of high sensory
input, a requirement of additional activity in areas of the limbic lobe may be
necessary to avoid non-relevant stimuli. The high limbic system activity
observed in the middle-aged subjects might be a compensatory mechanism that may
help to maintain an adequate level of internal processes related to episodic
memory, conceptual processing, stimulus-independent thought and self-reflection.
The increased beta2 connectivity found between anterior and central
areas belongs to the frontal lobe and may be a functional signature in a
network underlying the DMN. The high activity of the DMN likely helps to
maintain internal cognitive processes at an acceptable level in the middle-aged
subjects.
Brain activity in middle-aged vs. elderly
subjects
We found more alpha1 and alpha2 oscillations in frontal,
temporal, parietal and occipital areas, and fewer beta2 oscillations in
the limbic lobe in elderly subjects. No statistically significant changes in
functional connectivity were observed. The increase of resting alpha
oscillations suggests that, at rest, the elderly subjects have less neural
activity and more cortical inhibition than the middle-aged subjects. The main
regions affected by the relative decrease of neural activity were located at
the parietal lobe, namely precuneus and right inferior parietal lobe. Similar
increases in parietal alpha oscillations in normal elderly have
previously been reported in the literature [39]. Both precuneus and inferior
parietal lobe are regions involved in attention, memory and visuospatial
processing/interpretation. The relative cortical deactivation at rest may be
necessary to maintain an acceptable memory performance in normal non-demented
elderly subjects. This interpretation is probable if for cognitive processes
the initial deactivation of the cortex is helpful because it prevents
interferences in the highly selective access to memory trace [33].
Particularly, the increase in parietal alpha oscillations likely have a
main role in the conservation of an adequate cognitive outcome. Recent research
has found significant decreases in alpha oscillations at parietal lobe
in AD [18] linking the loss of parietal alpha activity
with cognitive decline. However, the relative cortical deactivation that
enables the maintenance of the cognitive state may induce an impairment in
perception performance in the elderly since the perception performance is in
fact enhanced if the cortex is activated before stimulus [33].
Together with the functional changes seen at neocortex, we found less activity
of the limbic lobe in the elderly. The hypoactivity of the limbic system may be
a cause of alterations in awareness, memory and behavior that are
characteristic of the elderly. In these individuals the episodic memory is
especially affected. Although the participants had no cognitive decline, in our
study we found that PCC (which plays a principal role in episodic memory) is
the region that shows the most significant loss of activity. The PCC forms a
central node in the DMN; thus, together with the results of the alpha activity,
our study suggests that DMN is less prominent in the elderly than in the
middle-aged subjects. The impairment of the DMN (namely, loss of limbic lobe
function and increased cortical inhibition affecting the parietal lobe) may be
seen as a biomarker of physiological aging. Alterations in the DMN in elderly
have been seen previously by other authors. Resting-state fMRI showed that
older subjects may recruit additional resources in frontal and temporal cortex
to compensate for these reductions in DMN [40]. We found no data in neural
activity that supports the involvement of additional cortical areas for the
DMN. We found, in fact, less neural activity in frontal and temporal lobe.
These opposed results may be due to the fact that fMRI studies reflect
age-related changes in neurovascular coupling not directly associated with
neural activity.
Brain activity in young vs. elderly subjects
Compared to young subjects, elderly individuals showed a significant
decrease in alpha2 oscillations in occipital lobe, mainly at the cuneus
(Figure 5). Similar decreases had
been seen by other authors in recent studies involving a slowing of occipital
activity in the elderly [6,7]. The impaired function of the
occipital lobe may be related to a reduction in grey matter volume and
disruptions of thalamo-cortical circuits. Supporting this, previous studies
have reported significant reductions in grey matter volume in the occipital
lobe in older subjects [27]. The relation between decreased
brain activity and decreased grey matter volume is well established in the
literature [41]. Recent research shows an
age-dependent decrease in thalamocortical synaptic transmission in healthy
elderly subjects. Some authors found an impaired phase synchronization between
thalamus and cuneus associated with alpha2 oscillations and increased
age [15]. Furthermore, the decrease in occipital alpha2
EEG sources might be associated with changes in the functioning of the
cholinergic basal forebrain system, which is supposed to induce a sustained
increase in excitatory activity in the cholinergic brainstem pathway, desynchronizing
the resting alpha rhythms at the cortical level and producing a mild
enhancement of cortical excitability [6].
In addition to the source localization results, our connectivity
analyses revealed significant decreases in LLC and LNC as measures of
functional connectivity, affecting the alpha2 frequency band (Figure 3). Decreased alpha2
connectivity may indicate a disruption in neural communication affecting
occipito-temporal and fronto-parietal circuits that occur in an intra- (O2-T8)
and inter- (O1-T8 and P4-F7) hemispherical manner. Interestingly, these
disconnections affect several regions that belong to the DMN. Recent research
using fMRI showed an equivalent decrease in magnitude of the DMN in the elderly
and its association with decline in the domains of
attention/concentration/processing speed, memory function and executive
functioning [27]. Our study shows that impaired
DMN function is mainly caused by an interhemispheric disconnection between left
prefrontal and right parietal cortex. Due to the principal role of the DMN in
functional organization of the brain, it is presumable that the stabilization
of brain ensembles, consolidation of the past and preparation for the future is
impaired in some degree in older people. The amount of task-unrelated thoughts
in this group is probably affected too, since the generation of spontaneous
thoughts is related to DMN magnitude [42,43]. The reduced efficiency of brain
networks observed, namely disruptions of long-range cortical circuits, may be
associated with physiological aging through attenuated dopamine transmission
[44] together with grey and white matter deficits
in frontal and temporal regions [45,46].
In conclusion, age induces non-linear regional variations in cortical
activity and disruptions in functional connectivity between specific brain
areas. The functional changes affect regions belonging to several resting-state
networks, including the DMN, and likely involve age-related differences in
attentional and cognitive processes. At middle-age, the main finding is a loss
of functional connectivity in occipito-temporal networks accompanied by an
increase in occipito-temporal and parietal cortical activity, together with an
increased magnitude of the DMN. In the elderly, in contrast, the frontal,
temporal, parietal and occipital cortical activity decreases. The decreased
cortical activity at rest, especially the increase in alpha1 and alpha2
activities at the parietal lobe, likely allows the conservation of a good
cognitive income, as indicated by recent research linking decreased parietal
alpha with cognitive impairment.
GENOTYPE-RELATED CHANGES IN
BRAIN ACTIVITY
Genomic factors potentially related to changes in brain bioactivity
include at least five categories of gene clusters: (1) genes associated with
disease pathogenesis (e.g. AGT in vascular dementia); (2) genes
associated with the mechanism of action of drugs; (3) genes associated with
drug metabolism (phase I and II reactions); (4) genes associated with drug
transporters; and (5) pleiotropic genes involved in multifaceted cascades and
metabolic reactions (e.g. APOE) [5].
APOE gene. The APOE-4 allele is associated with
genetic predisposition to suffering AD and with both AD-related abnormalities
in cortical rhythms and disintegration of functional connectivity pattern in AD
patients. Specific patterns of functional network disruption affecting theta
and alpha band associated with the level of cognitive disturbance or
with the APOE genotype have been found in AD [18]. Namely, AD patients had less
parieto-occipital alpha activity than controls, and those carrying the APOE-4
allele exhibited reduced alpha oscillations in left parietal and
temporo-occipital regions in comparison with noncarriers. The reduction in alpha
power found in patients with AD most likely represents disease- and
genotype-related resting-state regional dysfunction. There was a decreased alpha2
connectivity pattern in AD, involving the left temporal and bilateral parietal
cortex. Several regions exhibited increased lagged phase synchronization in the
theta band across and within hemispheres, where temporal lobe
connections were particularly compromised. In patients with early AD, there was
an APOE-4 allele-related decrease in interhemispheric alpha connectivity
in frontal and parietal regions.
Despite an increasing body of literature on APOE-brain
network relationship in AD, little is known about the influence of APOE
genotype on resting-state functional connectivity in cognitively healthy individuals. There are
controversial data concerning the impact of the APOE genotype on
cognitive functioning and brain activity in older healthy subjects. Some PET
and fMRI studies have shown that APOE-4 carriers have reduced activity
in the PCC, parieto-temporal and frontal cortex [47,48]. Other authors found altered connectivity between
regions implicated in the DMN and subcortical regions and recent studies have
shown increased connectivity between the DMN and hippocampus [49], and
better cognitive performance in healthy APOE-4 carriers [50]. To
investigate the potential APOE-4 allele influence on brain activity in
healthy elderly, we compared 12 APOE-4 carriers
and 28 non-carriers with no signs of cognitive deficit. The averaged eLORETA
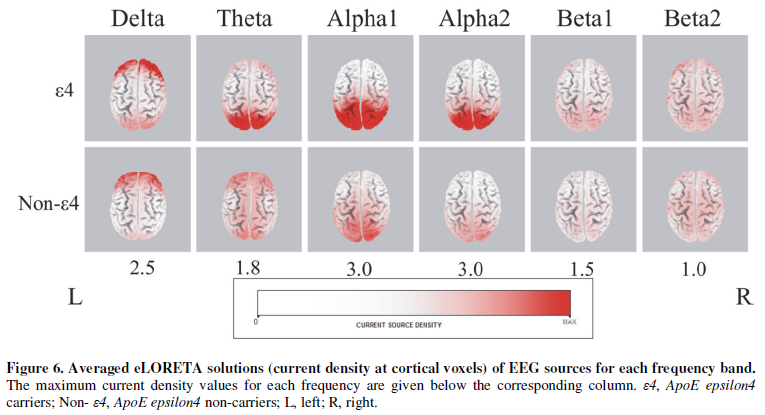
solutions show that the bioelectrical neural activity was higher in APOE-4
carriers compared to non-carriers in all frequency bands (Figure 6). Higher current density maxima
were found particularly in delta (APOE-4 carriers: 3.74, APOE-4
non-carriers: 2.98), theta (APOE-4 carriers: 2.3, APOE-4
non-carriers: 1.09), alpha1 (APOE-4 carriers: 7.21, APOE-4
non-carriers: 1.69) as well as in the alpha2 band (APOE-4
carriers: 4.01, APOE-4 non-carriers: 1.38). There was a similar cortical
distribution of maximal activity across groups; alpha1 and alpha2
activity were maximal in occipital regions. Delta and theta bands
were predominant in the prefrontal cortex; however the theta band had
maximum values in occipital cortex in APOE-4 carriers. Statistical
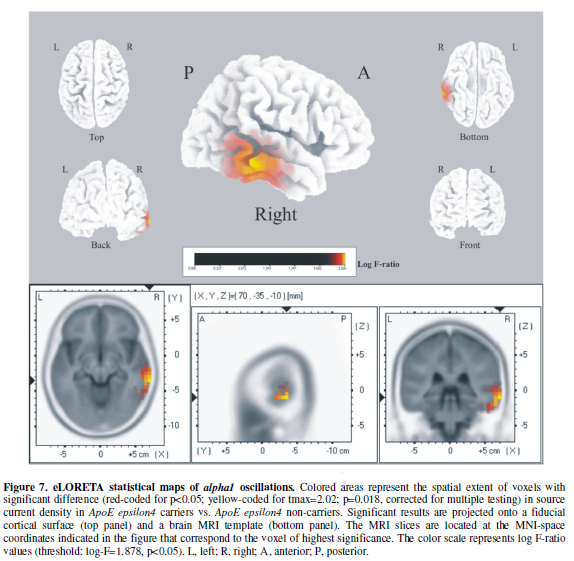
analysis revealed significant differences between groups exclusively in the alpha1
band. APOE-4 carriers exhibited significantly increased current density
in the alpha1 band in the right temporal cortex (Figure 7). APOE-4 carriers had significantly increased
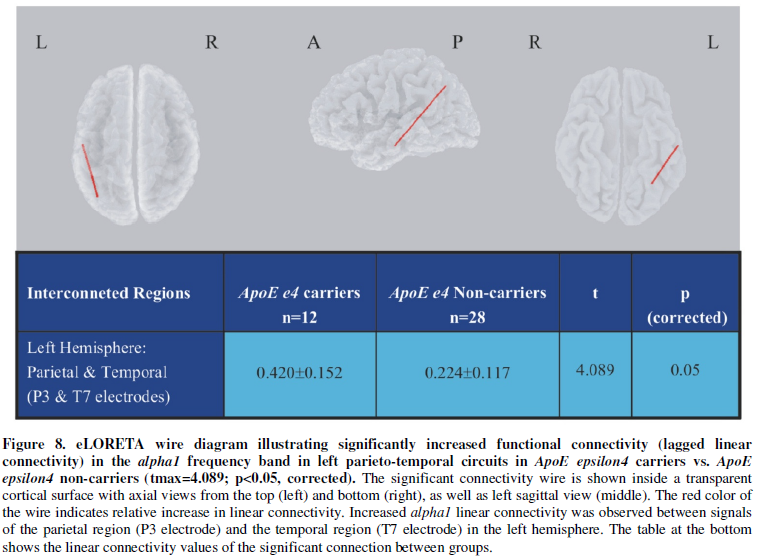
functional connectivity in the alpha1 band compared to non-carriers.
This increased connectivity was found in the left hemisphere between the
posterior parietal and temporal cortex (Figure
8). Both findings, namely more alpha1 in right temporal and more
connectivity in the alpha1 band between left parieto-temporal regions in
healthy elderly APOE-4 carriers, implies singular differences in
brain function associated with the APOE-4 allele. More alpha1
oscillations may indicate less activity in right temporal cortex (more alpha
is indicative of less cortical activity). The temporal lobe is a key region in
AD pathogenesis. Our data may be interpreted as a sign of cortical disturbance
in these subjects even in an asymptomatic stage. Interestingly,
since decreased connectivity in alpha range between parieto-temporal
regions is a trait observed in AD, our increased alpha1 connectivity in
the left hemisphere may be a potential compensatory mechanism that preserves a
good cognitive status in these individuals with genetic vulnerability. The
increased connectivity observed may be a primary stage of increased
connectivity in temporal regions, as observed by Canuet et al in AD patients.
Our findings may indicate that: (i) cortical dysfunction affects the right
temporal lobe, and (ii) some compensatory mechanism may involve
parieto-temporal resources in the left hemisphere.
AGT gene. The AGT gene belongs to the
renin-angiotensin system that regulates blood pressure and plays a principal
role in the control of vascular function. AGT is a key factor in the occurrence
and progression of vascular dementia (VD). VD is nowadays the second cause of
dementia in the world, after AD. In a recent work, our team investigated the
influence of two SNPs in the AGT gene (235T and 174M) associated with
arterial hypertension and cerebrovascular pathology, on brain activity in VD
patients [51]. We observed that VD patients with genomic risk (carriers of
allelic variants associated with vascular pathology) had more connectivity in delta
band between frontal, fronto-temporal and fronto-parietal regions. Our findings
show that in VD high blood pressure disturbs the functional connectivity at the
frontal level. The slow hyperconnectivity observed may be a direct reflection
of neural damage caused by high blood pressure in susceptible individuals.
CYPs:
Pharmacogenomic factors may account for 60-90% of drug variability in
drug disposition and pharmacodynamics. Approximately 60-80% of CNS drugs are
metabolized via enzymes of the CYP gene superfamily. About 57.76% of patients
with AD are extensive metabolizers (EMs) for CYP2D6 enzymes, 31.06% are
intermediate metabolizers (IMs), 5.28% are poor metabolizers (PMs), and 5.90%
are ultrarapid metabolizers (UMs); 73.71% are CYP2C19-EMs, 25.12% IMs, and
1.16% PMs; 60.87% are CYP2C9-EMs, 34.16% IMs, and 4.97% PMs; 82.75% are
CYP3A4/5-EMs, 15.88% IMs, and 1.37% UMs. A trigenic cluster integrating
CYP2D6+CYP2C19+CYP2C9 polymorphic variants yields 82 different haplotype-like
profiles, representing 36 different pharmacogenetic phenotypes in which only
26.51% of patients show a pure 3EM phenotype[52]. These data clearly indicate that
the incorporation of pharmacogenomic protocols to dementia research and
clinical trials can foster therapeutic optimization by helping to develop
cost-effective pharmaceuticals and improve drug efficacy and safety.
CYP2D6: The CYP2D6 gene is a genetically
polymorphic gene involved in the metabolism of several psychoactive drugs.
Recent studies show that CYP2D6 may be involved
in the production and biotransformation of neurotransmitters, such as
dopamine and serotonin, whose influence on brain function and behavior is well
established in the literature [53,54]. In individuals that have gene
variants that lead to a complete lack of functional enzyme (CYP2D6 poor
metabolizers) both hepatic and brain levels of CYP2D6 are reduced. Increased
anxiety and impulsivity have been associated with being a CYP2D6 poor
metabolizer [55]. Compared with CYP2D6
extensive metabolizers, poor metabolizers show a significant increase in the
activity of the thalamus and hippocampus, two regions with high expression of
CYP2D6 protein and mRNA [56].
CYP2D6 poor metabolizers are at a higher risk for
developing Parkinson’s disease (PD) [57], and this risk is further
increased when these individuals are exposed to pesticides [58].
This suggests that CYP2D6 poor metabolizers may be unable to
inactivate environmental toxins that increase the risk for developing PD.
CYP2D6 is expressed within PD-affected brain regions (for example within the
pigmented neurons of the substantia nigra) and is thus ideally situated to
participate in the local inactivation of PD-causing neurotoxins. In contrast,
inhibition of CYP2D6 in human neuroblastoma cells increased the neurotoxic
effects of neurotoxins [59].
CYP2C19: Genetic variation in CYP2C19,
involved in the metabolism of serotonin and oxidation of sexual hormones, such
as testosterone and progesterone, has also been associated with heritable
personality traits such as reward dependence, cooperativeness and
self-transcendence in females [60].
CYP2C9: It has recently been found that CYP2C9 gene polymorphism is associated
with phenytoin toxicity in infants with epilepsy [61] and with reductions in cerebellar
volume in epileptic users of phenytoin [62].
CYP3A4: The induction of CYP3A4 in the brain
has been associated with cognitive and behavioral dysfunction. Potent inducers
of CYP3A4 in the brain, such as anti-epileptic drugs (e.g.
oxcarbazepine, carbamazepine and phenytoin) increase the metabolism of
testosterone and estradiol, which are involved in mood, behavior, sexuality,
memory and cognition [63]. The endocrine dysfunction
associated with the induction of CYP3A4 illustrates how brain CYPs
can potentially modulate the local concentrations of endogenous molecules and
affect brain function and behavior. Another example is the CYP1A1- and CYP1A2-mediated
metabolism of arachidonic acid in the brain, which produces epoxyeicosatrienoic
acid (ETTs) and hydroxyeicosatetraenoic acids (HETEs) known to participate in
critical biological processes, such as calcium signaling, vesicle release and
the vasodilation of cerebral arteries [64].
Drug-induced brain toxicity is a typical
finding in carriers of CYP3A4 mutant variants associated with poor drug
metabolism. Particularly, neurotoxicity has been found in therapies with
anti-epileptic or anti-tumoral agents [65,66]. EEG is useful to
detect early cortical dysfunctions associated with neurotoxicity, such as,
abnormal beta activity, aberrant connectivity patterns, paroxysmal patterns and
epilepto form discharges. EEG is a powerful tool to investigate the action and
safety of drugs on CNS. However, a review of the literature reveals
inconsistent operating procedures from one study to another. While this fact
does not invalidate results per se, the lack of standardization constitutes a
regrettable shortcoming, especially in the context of drug development programs
[67]. The incorporation of pharmacogenetic programs to drug development and
clinical drug assessment (efficacy and safety) may help to optimize
therapeutics as well as the utility of brain mapping as a biomarker. It has
been clearly demonstrated that in patients with dementia APOE-4 carriers
are poor responders to conventional treatments [68], and a good correlation has
been found with EEG parameters in these patients [69,70].
CONCLUSION
Age and genotype dramatically influence brain function. We found that
age induces non-linear changes in cortical activity and functional disruptions
in brain networks. Functional changes affect regions that belong to several
resting-state networks, including the DMN, and likely involve age-related
differences in attentional and cognitive processes. The APOE gene is a
key gene for brain activity despite the predisposition to suffer AD. We found
significant differences in brain function associated with the APOE-4
allele even in healthy subjects. We also found that VD patients with genomic
risk (carriers of AGT variants associated with vascular pathology) show
disturbances in functional connectivity at the frontal level. Finally, we
propose that the CYP superfamily may also play a possible role in brain
activity.
ACKNOWLEDGEMENTS
This work was supported by EuroEspes and the International Agency for
Brain Research and Aging (IABRA). The authors would like to thank the nurses
Margarita Alcaraz and Laura Nebril for their collaboration in blood sampling as
well as Yolanda González and Lucía López for their help with MRI studies.
DISCLOSURE STATEMENT
There are no actual or potential conflicts of interest including any
financial, personal or other relationship with other people or organizations
that could inappropiately influence this work.
- Burke
SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7: 30-40.
- Kanchibhotla
SC, Mather KA, Wen W, Schofield PR, Kwok JB, et al. (2013) Genetics of
ageing-related changes in brain white matter integrity - a review. Ageing Res Rev 12: 391-401.
- Peters
R (2006) Ageing and the brain. Postgrad
Med J 82: 84-88.
- Cacabelos
R, Torrellas C (2015) Epigenetics of Aging and Alzheimer's Disease:
Implications for Pharmacogenomics and Drug Response. Int J Mol Sci 16: 30483-30543.
- Cacabelos R, Torrellas C, Carrera I, Cacabelos P,
Corzo L, et al. (2016)
Novel Therapeutic Strategies for Dementia. CNS Neurol Disord Drug Targets 15: 141-241.
- Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del
Percio C, et al. (2006)
Sources of cortical rhythms in adults during physiological aging: a
multicentric EEG study. Hum Brain
Mapp 27: 162-172.
- Davidson
PN, Davidson KA (2012) Electroencephalography in the elderly. Neurodiagn J 52: 3-19.
- Olbrich
S, van Dinteren R, Arns M (2016) Personalized Medicine: Review and
Perspectives of Promising Baseline EEG Biomarkers in Major Depressive
Disorder and Attention Deficit Hyperactivity Disorder. Neuropsychobiol 72:
229-240.
- Alvarez XA, Mouzo R, Pichel V, Pérez P, Laredo M, et
al. (1999)
Double-blind placebo-controlled study with citicoline in APOE genotyped
Alzheimer's disease patients. Effects on cognitive performance, brain
bioelectrical activity and cerebral perfusion. Methods Find Exp Clin Pharmacol 21: 633-644.
- Alvarez
XA, Sampedro C, Figueroa J, Tellado I, González A, et al. (2008)
Reductions in qEEG slowing over 1 year and after treatment with Cerebrolysin
in patients with moderate-severe traumatic brain injury. J Neural Transm 115: 683-692.
- Gorbachevskaya
N, Bashina V, Gratchev V, Iznak A (2001) Cerebrolysin therapy in Rett
syndrome: clinical and EEG mapping study. Brain Dev 23: S90-S93.
- Buckner
RL, Vincent JL (2007) Unrest at rest: default activity and spontaneous
network correlations. Neuroimage 37:
1091-1096.
- Raichle
ME, Snyder AZ (2007) A default mode of brain function: a brief history of
an evolving idea. Neuroimage
37: 1083-1090.
- Pascual-Marqui
RD (2007) Discrete, 3D distributed, linear imaging methods of electric
neuronal activity. Part 1: exact, zero error localization. arXiv:0710.3341
[math-ph].
- Cantero JL, Atienza M, Gomez-Herrero G, Cruz-Vadell
A, Gil-Neciga E, et al. (2009) Functional integrity of thalamocortical circuits
differentiates normal aging from mild cognitive impairment. Hum Brain Mapp 30: 3944-3957.
- Mesulam
M (2004) The cholinergic lesion of Alzheimer's disease: pivotal factor or
side show?. Learn Mem 11:
43-49.
- Babiloni C, Binetti G, Cassetta E, Cerboneschi D,
Dal Forno G, et al. (2004) Mapping distributed sources of cortical rhythms in mild
Alzheimer's disease. A multicentric EEG study. Neuroimage 22: 57-67.
- Canuet
L, Tellado I, Couceiro V, Fraile C, Fernandez-Novoa L, et al. (2012) Resting-state
network disruption and APOE genotype in Alzheimer's disease: a lagged
functional connectivity study. PLoS One 7: e46289.
- Dierks
T, Ihl R, Frölich L, Maurer K (1999) Dementia of the Alzheimer type:
effects on the spontaneous EEG described by dipole sources. Psychiatry Res. 50: 151-162.
- Jelic
V, Shigeta M, Julin P, Almkvist O, Winblad B, et al. (1996) Quantitative
electroencephalography power and coherence in Alzheimer's disease and mild
cognitive impairment. Dementia 7: 314-323.
- Jelic
V, Johansson SE, Almkvist O, Shigeta M, Julin P, et al. (2000)
Quantitative electroencephalography in mild cognitive impairment:
longitudinal changes and possible prediction of Alzheimer's disease. Neurobiol Aging 21: 533-540.
- Allen
JJ, Coan JA, Nazarian M (2004) Issues and assumptions on the road from raw
signals to metrics of frontal EEG asymmetry in emotion. Biol Psychol 67: 183-218.
- Davidson
RJ (1988) EEG measures of cerebral asymmetry: conceptual and
methodological issues. Int J
Neurosci 39: 71-89.
- Knyazev
GG, Slobodskoj-Plusnin JY, Bocharov AV, Pylkova LV (2011) The default mode
network and EEG α oscillations: an independent component analysis. Brain Res 1402: 67-79.
- Mantini
D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M (2007) Electrophysiological
signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104:
13170-13175.
- Bakkour
A, Morris JC, Wolk DA, Dickerson BC (2013) The effects of aging and
Alzheimer's disease on cerebral cortical anatomy: specificity and differential
relationships with cognition. Neuroimage
76: 332-344.
- Damoiseaux
JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, et al. (2008) Reduced
resting-state brain activity in the "default network" in normal
aging. Cereb Cortex 18:
1856-1864.
- Matousek
M, Brunovsky M, Edman A, Wallin A (2001) EEG abnormalities in dementia
reflect the parietal lobe syndrome. Clin
Neurophysiol 112: 1001-1005.
- Sheridan
PH, Sato S, Foster N, Bruno G, Cox C, et al. (1988) Relation of EEG alpha
background to parietal lobe function in Alzheimer's disease as measured by
positron emission tomography and psychometry. Neurology 38: 747-750.
- Klimesch
W (1999) EEG alpha and theta oscillations reflect cognitive and memory
performance: a review and analysis. Brain
Res Brain Res Rev
29: 169-195.
- Köpruner
V, Pfurtscheller G, Auer LM (1984) Quantitative EEG in normals and in
patients with cerebral ischemia. Prog
Brain Res 62: 29-50.
- Laufs
H, Krakow K, Sterzer P, Eger E, Beyerle A, et al. (2003)
Electroencephalographic signatures of attentional and cognitive default
modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 100:
11053-11058.
- Klimesch
W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations: the
inhibition-timing hypothesis. Brain
Res Rev 53: 63-88.
- Mathewson
KE, Lleras A, Beck DM, Fabiani M, Ro T, et al. (2011) Pulsed out of
awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing
cortical processing. Front
Psychol 2: 99.
- Ergenoglu
T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, et al. (2004) Alpha
rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res 20:
376-383.
- Klimesch
W, Vogt F, Doppelmayr M (2000) Interindividual differences in alpha and
theta power reflect memory performance. Intelligence 27: 347-362.
- Vogt
F, Klimesch W, Doppelmayr M (1998) High-frequency components in the alpha
band and memory performance. J
Clin Neurophysiol 15: 167-172.
- Doppelmayr
M, Klimesch W, Stadler W, Pöllhuber D, Heine C (2002) EEG alpha power and
intelligence. Intelligence 30:
289-302.
- Breslau
J, Starr A, Sicotte N, Higa J, Buchsbaum MS (1989) Topographic EEG changes
with normal aging and SDAT. Electroencephalogr
Clin Neurophysiol 72: 281-289.
- Greicius
MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity
distinguishes Alzheimer's disease from healthy aging: evidence from
functional MRI. Proc Natl Acad
Sci USA 101: 4637-4642.
- Johnson
SC, Saykin AJ, Baxter LC, Flashman LA, Santulli RB, et al. (2000) The
relationship between fMRI activation and cerebral atrophy: comparison of
normal aging and alzheimer disease. Neuroimage
11: 179-187.
- Raichle
ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. (2001) A default
mode of brain function. Proc Natl
Acad Sci USA 98: 676-682.
- Mason
MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, et al. (2007) Wandering
minds: the default network and stimulus-independent thought. Sci 315: 393-395.
- Achard
S, Bullmore E (2007) Efficiency and cost of economical brain functional
networks. PLoS Comput Biol 3: e17.
- Scahill
RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, et al. (2003) A
longitudinal study of brain volume changes in normal aging using serial
registered magnetic resonance imaging. Arch Neurol 60: 989-994.
- Head
D, Buckner RL, Shimony JS, et al. (2004) Differential vulnerability of
anterior white matter in nondemented aging with minimal acceleration in
dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex 14: 410-423.
- Reiman,
EM, Chen K, Alexander GE, et al. (2004) Functional brain abnormalities in
young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 101:
284-289.
- Reiman,
EM, Chen K, Alexander GE, Caselli RJ, Bandy D, et al. (2005) Correlations
between apolipoprotein E epsilon4 gene dose and brain-imaging measurements
of regional hypometabolism. Proc
Natl Acad Sci USA 102: 8299-8302.
- Westlye
ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT (2011) Increased
hippocampal default mode synchronization during rest in middle-aged and
elderly APOE ε4 carriers: relationships with memory performance. J Neurosci 31: 7775-7783.
- Alexander
DM, Williams LM, Gatt Dobson-Stone C, Kuan SA, Todd EG, et al. (2007) The
contribution of apolipoprotein E alleles on cognitive performance and
dynamic neural activity over six decades. Biol Psychol 75: 229-238.
- Tellado I, Carril JC, Couceiro V, Fraile C,
Torrellas C, et al. (2015) Impacto del genotipo AGT sobre la actividad
cerebral en la demencia vascular. Gen-T
10: 137-142.
- Cacabelos
R (2010) Pharmacogenomic protocols in CNS disorders and dementia. Neurodegener Dis 7: 167-169.
- Bromek
E, Haduch A, Gołembiowska K, Daniel WA (2011) Cytochrome P450 mediates
dopamine formation in the brain in vivo. J Neurochem 118: 806-815.
- Yu
AM, Idle JR, Byrd LG, Krausz KW, Küpfer A, et al. (2003) Regeneration of serotonin from
5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics 13: 173-181.
- Peñas-LLedó
EM, Dorado P, Pacheco R, González I, LLerena A (2009) Relation between
CYP2D6 genotype, personality, neurocognition and overall psychopathology
in healthy volunteers. Pharmacogenomics 10: 1111-1120.
- Kirchheiner J, Seeringer A, Godoy AL,
Ohmle B, Maier C, et al. (2011) CYP2D6 in the brain: genotype effects on
resting brain perfusion. Mol
Psychiatry 16: 333-341.
- McCann SJ, Pond SM, James KM, Le Couteur DG (1997) The association
between polymorphisms in the cytochrome P-450 2D6 gene and Parkinson's
disease: a case-control study and meta-analysis. J Neurol Sci 153:
50-53.
- Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F,
et al. (2004)
CYP2D6 polymorphism, pesticide exposure, and Parkinson's disease. Ann Neurol 55: 430-434.
- Mann
A, Tyndale RF (2010) Cytochrome P450 2D6 enzyme neuroprotects against
1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci 31: 1185-1193.
- Ishii
G, Suzuki A, Oshino S, Shiraishi H, Otani K (2007) CYP2C19 polymorphism
affects personality traits of Japanese females. Neurosci Lett 411: 77-80.
- Veeravigrom
M, Jaroonvanichkul V, Netbaramee W, Phaisarn P, Uyathanarat T (2016)
Phenytoin toxicity in two-month-old Thai infant with CYP2C9 gene
polymorphism - A case report. Brain
Dev 38: 136-138.
- Twardowschy
CA, Werneck LC, Scola RH, Borgio JG, De Paola L, et al. (2013) The role of
CYP2C9 polymorphisms in phenytoin-related cerebellar atrophy. Seizure 22: 194-197.
- McEwen BS (1994) How do sex and stress hormones affect nerve cells?. Ann N Y Acad Sci 743: 1-16..
- Rifkind
AB (2006) CYP1A in TCDD toxicity and in physiology-with particular
reference to CYP dependent arachidonic acid metabolism and other
endogenous substrates. Drug Metab
Rev 38: 291-335.
- de
Graan AJ, Elens L, Sprowl JA, Sparreboom A, Friberg LE, et al. (2013)
CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced
neurotoxicity. Clin Cancer Res 19:
3316-3324.
- Ghosh
C, Hossain M, Spriggs A, Ghosh A, Grant GA, et al. (2015)
Sertraline-induced potentiation of the CYP3A4-dependent neurotoxicity of
carbamazepine: an in vitro study. Epilepsia
56: 439-449.
- Jobert
M, Wilson FJ, Ruigt GS, Brunovsky M, Prichep LS, et al. (2012) Guidelines
for the recording and evaluation of pharmaco-EEG data in man: the
International Pharmaco-EEG Society (IPEG). Neuropsychobiol 66:
201-220.
- Cacabelos
R, Cacabelos P, Torrellas C, Tellado I, Carril JC (2014) Pharmacogenomics
of Alzheimer’s disease: novel therapeutic strategies for drug development.
Methods Mol Biol 1175: 323-556.
- Cacabelos R, Alvarez XA, Lombardi V, Fernández-Novoa
L, Corzo L, et al. (2000)
Pharmacological treatment of Alzheimer’s disease: From psychotropic drugs
and cholinesterase inhibitors to pharmacogenomics. Drugs Today 36: 415-499.
- Cacabelos R, Alvarez XA, Fernandez-Novoa L, Lombardi
VRM (2000) A
pharmacogenomic approach to Alzheimer’s disease. Acta Neurol Scand Suppl. 102:12-19.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Astronomy and Space Research
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)