653
Views & Citations10
Likes & Shares
A study was undertaken to understand the genetics of cotton fibre development at University of Agricultural Sciences, Dharwad. Segregating F2 populations of the cross between fibred MCU-5 (WT) × fibreless mutant MCU-5 (fl) and its reciprocal cross of upland cotton (Gossypium hirsutum L.) were evaluated to study the inheritance of the fibre development. In both, straight and reciprocal crosses the segregation was in the ratio of 15 (fibred): 1(fibreless), indicating duplicate dominant gene interaction signifying that the fibreless trait is controlled by double recessive genes. However, among the fibred plants presence of genetic variability for number of fibres per unit area was observed. It infers that, the presence of individual cell level control over its elongation as a fibre. Scanning electron microscopy (SEM) was performed at fibre initiation stage, 2 DPA, to observe the development of fibre initials on epidermal layer of ovules. As compared to WT, SEM analysis revealed that the presence fibre cell initials in fl mutant found negligible.
Keywords: Fibre initiation, Fibre development, Fibreless mutant
Abbreviations: DPA: Days Post Anthesis; fl: fuzzless-lintless; SEM: Scanning Electron Microscope; WT: Wild Type
INTRODUCTION
Cotton fibres are unicellular trichomes originating from the outer epidermal layer of the seed coat. About 20 to 25% of the seed epidermal cells differentiate into spinnable fibres [1-4]. Fibres are classified into two types, lint and fuzz. Fibre development includes four distinct, but overlapping stages namely initiation, elongation/primary cell wall (PCW) synthesis, secondary cell wall (SCW) synthesis and maturation. The lint fibres initiate growth between anthesis and 2 days postanthesis (DPA) and can elongate 2.5-3.5 cm. Whereas, the fuzz fibres initiate growth between 5 and 10 DPA and are approximately 0.5 cm [4]. However, fast elongation of fibre cell occurs between 5 to 15 DPA. Secondary cell wall synthesis starts at about 20 DPA and continues up to 45 DPA. During this period large amount of cellulose (>90%) deposition takes place and the fibre cell wall becomes thick. In the final maturation stage (45-50 DPA) fibres undergo dehydration and produce mature cotton lint [1,5]. Spontaneous fibreless mutants of cotton have been reported since 1920 [6,7]. They were used to detect and locate QTLs for lint yield [8], fibre quality [9], seed traits [10], pattern of lint initiation [11], to determine differences in gene/protein regulation during development of ovular trichomes, for studying gene functions [12]. Lint yield depends on number of bolls per unit area, number of seeds per boll, number of fibres per seed, number of fibres per unit area and average weight per fibre. Improvements in fibre number per seed and per unit area of seed might results in increased lint yield. Thus, in the present study from the cross WT × fl and fl × WT an attempt was been made to understand the variation for number of fibres per unit area in segregating F2 population besides genetics of fibre development.
MATERIAL AND METHODS
Number of fibers/sample=Lint weight (g) × 1,000,000,000 (correction factor) ÷ Fibre fineness (mT) × Mean length of fibre (mm)
Number of fibers per seed=Number of fibers per sample ÷ Number of seeds per sample
Number of fibres per mm2=Number of fibres per seed ÷ Surface area of seed (mm2)
Scanning electron microscopy
At fibre initiation stage (2 DPA) scanning electron microscopy (SEM) was performed to observe the development of fibre initials on epidermal layer of ovules. Ovules excised from two days post anthesis flowers collected in 3% glutaraldehyde and dipped in 1-2% osmium tetroxide for post-fixation. Samples were dehydrated in a graded acetone solution and mounted on stub and allowed for metal coating with Gold using polaron sputter (E5100, Watford England) coater. The SEM images were captured by LEO 435 VP (LEO Electron microscopy Ltd., Cambridge, UK) at 15 kV EHT at Central Food Technological Research Institute (CFTRI), Mysore. The number of fibre initials was quantified in 100 µm2 area using ImageJ software.
RESULTS AND DISCUSSION
Number of fibre initials in parents
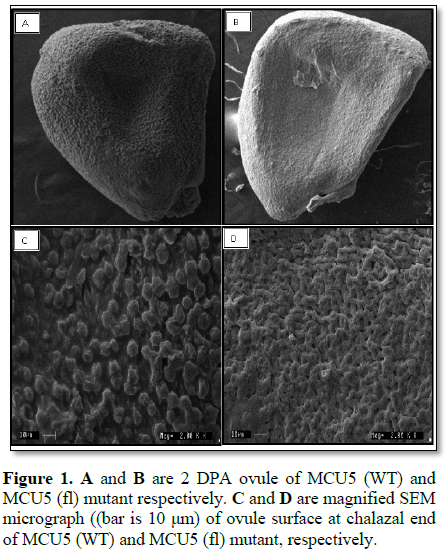
The literature says that fibre initiation starts from epidermal layer between -2 DPA to +2 DPA [5,15,16]. The cells which show elongation of initials by 2 DPA were further elongate into either fuzz (0.5 cm) or lint (2.5 to 3.5 cm). Only 20% of such initials going to elongate into fibres [1-4]. In the present study fibre initials were counted at 2 DPA stage. MCU5 (WT) normal fibred parent recorded 118 fibre initials per 100 µm2 whereas, fibre less mutant MCU (fl) found negligible fibre initials that is only two fibre initials per 100 µm2, indicating fibre less trait in mutant and scanning electron micrograph of 2 DPA ovules of both genotypes are depicted in Figure 1.
Genetics of fibre development in tetraploid cotton
The F1's obtained from both straight (WT × fl) and reciprocal (fl × WT) cross showing only fibred, indicates that fibred trait is governed by dominant genes. In the direct cross 749 and 51 F2 plants, and in reciprocal cross 797 and 55 F2 plants were recorded fibred and fibre less traits, respectively. This observed results from both cross F2 populations followed 15 (fibred):1(fibreless) segregation ratio and it was confirmed by chi-square test (Table 1). This indicates that the presence of duplicate dominant gene interaction and fibreless trait governed by two recessive genes. Similarly, Kumar et al. [17] concluded double recessive genes controls the lintless trait and they also reported presence of duplicate dominant gene action, from the study of inheritance pattern for fibreless trait in the diploid F2 populations of cross between Fuzz linted (FL) × Fuzz-lintless (fl) mutant. Nadarajan and Rangaswamy [18] studied the inheritance pattern for fibreless trait in F2 populations by crossing fibreless line with five different G. hirsutum lines. They observed 15(F):1(fls), 63(F):1(fls) and 255(F):1(fls) segregation ratios and concluded fibred trait controlled by two to four genes.
Variability for ginning outturn in tetraploid cotton
Presence variation for ginning outturn was recorded in F2 of crosses, MCU5 (WT) × MCU5 (fl) mutant and its reciprocal cross (fl × WT). The ginning outturn was in the range of 0 to 43.75% in WT × fl population and 0.77 to 43.90% in fl × WT F2 population (Figure 2). It was also evident from the visual observation of seeds being covered by number of fibres. As the number of fibres per seed increased, the increase in ginning outturn was noticed. For example, seeds of plant with ginning outturn 0% were not covered by any fibres, whereas the seeds of plant with ginning outturn of 9% were partially covered by fibres with 1525.90 fibres per seed and plant with ginning outturn 36.66% was fully covered by fibres with 20631.46 fibres per seed (Table 2). The ginning outturn recorded higher positive correlation with number of elongated fibres per seed (0.949), fibres per unit area (0.952) compared to surface area of seed (0.213) (Table 3 and 4). These results indicated that, as the increase in number of fibres per unit area there is increase in GOT percent. Previously, Li et al. [19] suggested that the number of fibre initials can be used as predictor of lint percentage in cotton breeding. Similarly Gabriela et al. [20] reported that fibre initial density alone can be used as easy and early predictor of lint percent or potential yield. In normal fibred cotton genotype the potential cells are elongating into fibre is said to be about 20 to 25% [1-4]. Bechere et al. [14] revealed that the number of fibres per seed and fibre density were lower in 9023n4 mutant than in the wild type, which explains low lint yield in mutant line. Thus, it is evident that the number of elongated fibres determines the ginning outturn and in turn it is possible to increase the ginning outturn percent by increasing number of elongating fibres per unit area.
1. Arpat AB, Waugh M, Sullivan JP, Gonzales M,
Frisch D, et al. (2004) Functional genomics of cell elongation in developing
cotton fibres. Plant Mol Biol 54: 911-929.
2. Wilkins TA, Arpat AB (2005) The cotton fibre
transcriptome. Physiol Plant 124: 295-300.
3. Hee KM, Barbara AT (2001) Cotton fibre growth
in planta and in vitro models for
plant cell elongation and cell wall biogenesis. Plant Physiol 127: 1361-1366.
4. Stewart JD (1975) Fibre initiation on the
cotton ovule. Am J Bot 62: 723-730.
5. Wu Y, Machado AC, White RG, Llewellyn DJ,
Dennis ES (2006) Expression profiling identifies genes expressed early during
lint fibre initiation in cotton. Plant Cell Physiol 47: 107-127.
6. Kohel RJ (1973) Genetic nomenclature in
cotton. J Heredity 64: 291-295.
7. Endrizz JE, Turcotte L, Kohel RJ (1984)
Qualitative genetics, cytology and cytogenetics. In: Kohel RJ and Lewis CF,
Eds., Cotton, Agronomy Society of America, Madison, pp: 59-80.
8. An C, Jenkins JN, Jixiang W, Guo Y, Mc-Carty
JC (2010) Use of fibre and fuzz mutants to detect QTL for yield components,
seed, and fibre traits of upland cotton. Euphytica 172: 21-34.
9. Paterson AH, Saranga Y, Menz M, Jiang CX,
Wright RJ (2010) QTL analysis of genotype × environment interactions affecting
cotton fibre quality. Theor Appl Genet 106: 384-396.
10. Song XL, Zhang TZ (2007) Identification of
quantitative trait loci controlling seed physical and nutrient trait in cotton.
Seed Sci Res 17: 243-251.
11. Turley RB, Kloth RH (2002) Identification of a
third fuzzless seed locus in upland cotton (Gossypium
hirsutum L.). J Heredity 93.
12. Rong JR, Pierce GJ, Waghmore VN, Rogers CJ,
De SA, et al. (2005) Genetic mapping and comparative analysis of seven mutants
related to seed fibre development in cotton. Theor Appl Genet 111: 1137-1146.
13. Peter SD, Muralidharan V, Vaman MB (1984)
Fuzzless-lintless mutant of MCU 5 cotton. Cotton Dev 14: 43.
14. Bechere E, Turley RB, Auld DL, Zeng L (2012)
A new fuzzless seed locus in an upland cotton (Gossypium hirsutum L.) Mutant Am J Plant Sci 3: 799-804.
15. Padmalatha K, Patil D, Kumar K, Dhandapani G,
Kanakachari M, et al. (2012) Functional genomics of fuzzless-lintless mutant of
Gossypium hirsutum L. cv. MCU5 reveal
key genes and pathways involved in cotton fibre initiation and elongation. BMC
Genomics 13: 624.
16. Liu K, Han M, Zhang C, Yao L, Sun J, et al. (2012)
Comparative proteomic analysis reveals the mechanisms governing cotton fibre differentiation
and initiation. J Proteomics 75: 845-856.
17. Kumar GS, Kumar NVM, Katageri IS (2017)
Genetics of fibre development in diploid cotton. Int J Curr Microbiol Appl Sci
6: 453-457.
18. Nadarajan N, Rangaswamy SRS (1988)
Inheritance of fuzzless-lintless in cotton (Gossypium
hirsutum L.). Theor Appl Genet 75: 728-730.
19. Li CW, Guo, Zhang T (2008) Fibre initiation
development in upland cotton (Gossypium
hirsutum L.) cultivars varying in lint percentage. Euphytica 165: 223-230.
20. Gabriela B, Romano EW, Turley TRB, Jodi AS (2011)
Fibre initiation in 18 cultivars and experimental lines of three Gossypium
species. J Cotton Sci 15: 61-72.a
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Astronomy and Space Research
- Proteomics and Bioinformatics (ISSN:2641-7561)