916
Views & Citations10
Likes & Shares
Introduction: Cryolipolysis is a well-tolerated
nonsurgical procedure in which controlled cooling is used to treat localized
fat. Investigators have also observed skin tightening as a result of this
treatment. However, scholars have not studied the extent to which cryolipolysis
influences the skin’s biomechanical characteristics.
Objective: This study was intended to provide
quantitative measurements of skin tightness after cryolipolysis treatment and
to evaluate that treatment’s effectiveness in the reduction of localized
subcutaneous fat.
Methods: In the study, we treated 21 subjects with
mean (SD) age 34 (9) years with cryolipolysis in the abdomen and flanks. We
performed evaluated the subjects’ anthropometry, took standardized photographs
and measured skin firmness. We assessed the subjects’ fat layers using skin
fold calipers and diagnostic ultrasound. We performed measurements at baseline
and followed up at 30, 60 and 90 days. The level of significance was P<0.05.
Results: There were no significant differences in body
weight or BMI between pretreatment and post treatment. The results of the
skin-firmness parameter analysis revealed significant differences between the
post treatment measurements at 30, 60 and 90 days, as compared to the
pretreatment measures. Cryolipolysis reduced the thickness of the fat layer and
thus decreased the waist-circumference, caliper and diagnostic ultrasound
measurements.
Conclusion: Although the exact mechanisms through which
cryolipolysis affects the skin remain unknown, our cutometer measurements
demonstrated that this treatment improved skin tightness in the treated areas
and reduced fat-layer thickness.
Keywords: Skin firmness, Cutometer, Fat reduction, Adipocyte, Adipose tissue,
Cooling
INTRODUCTION
Cryolipolysis is a popular, well-tolerated
nonsurgical procedure in which controlled cooling is used to selectively damage
fat cells and consequently induce apoptosis [1-4]. Several research groups have
demonstrated that fat cells, as compared to water-rich tissues such as the
skin, are more sensitive to cold [1-4]. The cryolipolysis technique is
performed using a cup-shaped applicator that induces a vacuum to draw the
target area into the applicator and position it between two cooling panels. The
vacuum reduces blood flow in the treated area; the constriction of blood
vessels accelerates the cooling process [5]. Between the skin and the inner
surface of the applicator is a thin membrane of tissue; this tissue is covered
with antifreeze lotion to protect the skin and ensure that the opposing
applicator plates completely couple [1,6]. The cooling is usually maintained
for 45 to 60 min [7]. Clinical researchers have investigated the safety and
efficacy of cryolipolysis treatments for subcutaneous fat reduction in several
areas of the body, including the abdomen, flanks, inner thighs, outer thighs,
arms, chest and sub mental fat [8-14]. Some investigators have even observed an
additional, unexpected benefit of cryolipolysis: skin tightening, which leads
to improved skin texture and laxity [15,16]. However, we found no studies on
the extent to which cryolipolysis influences the skin’s biomechanical characteristics. Therefore,
the purposes of
MATERIALS
AND METHODS
We performed this prospective study (with
pre- and post-intervention analysis) by treating subjects with cryolipolysis at
the Clinical Laboratory of the Ibramed Center for Education and Advanced
Training – CEFAI (Amparo, São Paulo, Brazil). All subjects signed
informed-consent forms, and we performed the treatments in accordance with the
Declaration of Helsinki. The Research Ethics Committee for Institutions,
Teaching and Research approved the study (CAAE: 61499416.5.0000.5490).
The 21 volunteers who took part in the study
had a mean (SD) age of 30 (7) years and an age range of 18 to50 years; 18 (85%)
were female. The inclusion criteria were the presence of localized subcutaneous
fat in the abdominal and flanks regions and a body mass index (BMI) <30. The
exclusion criteria were aesthetic treatment in the treatment areas in the
preceding 6 months; current cutaneous diseases in the treatment areas; current
systemic diseases; current pregnancy, lactation, or intention to become
pregnant; and history of cryoglobulinemia, cold urticaria or paroxysmal cold
hemoglobinuria.
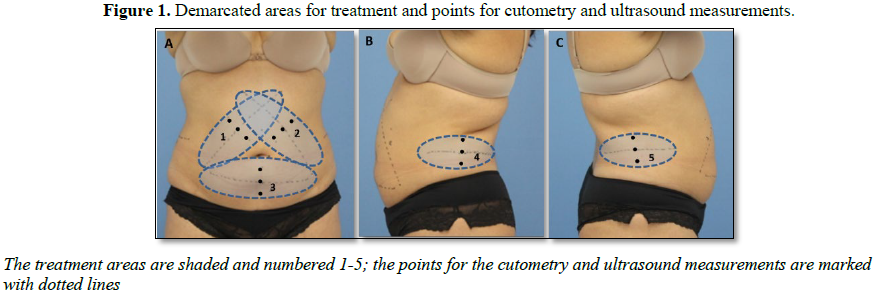
We identified, assessed, marked, and treated
the treatment sites with a conventional cryolipolysis Polaris
device (Ibramed, IndústriaBrasileira de EquipamentosMédicos - Amparo, São
Paulo, Brazil). We used a template to draw markings on the patients that would
guide the measurements and performed the treatments using medium (~300 cm3)
or large (~700 cm3) applicators, based on the size of the localized
fat areas and the anatomical limitations of the applicators’ placement. We
exposed each treated to -8°C for 60 min [17]. All participants underwent anthropometric
measurements for skin firmness, waist circumference and abdominal fat-layer
thickness. A trained physiotherapist took all measurements, and, for each
subject, the same physiotherapist performed the evaluations for every visit.
The evaluations occurred at pretreatment and at three follow-ups: 30, 60 and 90
days post treatment. We assessed the individuals’ heights and weights using a
classical mechanical stadiometer (110 CH Model, Welmy, SP, Brazil and measured
the circumferences of their abdomens using flexible measuring tape. To evaluate
skin firmness, we used a Cutometer MPA 580 (Courage & Khazaka Electronic,
Köln, Germany) to probe a 2 mm hole at 500 mbar of suction per second.
We performed the measurements in triplicate
and based the analysis on the means of these values. Before
each set of measurements, the volunteers spent 20 min in a closed environment
at a constant temperature (18°C to 22°C) and controlled relative humidity (55%
to 65%). The cutometer generated graphs that depicted immediate deformation
or skin extensibility (Ue), delayed distension (Uv),
final deformation (Uf), immediate retraction (Ur), total
recovery (Ua), and residual deformation at the end of the measuring
cycle (R). From these values, we computed two fractions: Uv/Ue,
the viscous component of the skin, and Ur/Uf, the
biological elasticity [18,19]. We also used the parameter R0=Uf
to refer to skin firmness or dispensability; R0 is one of the most important parameters [20].
We used skin fold calipers (RMC, Amparo, SP,
Brazil) to measure the point of greatest thickness within each treatment area.
We pulled the folds vertically and measured the thickness 2 cm to the side of
the umbilicus.
We performed the diagnostic ultrasound
assessments using a linear transducer with frequency of 6 MHz to 18 MHz
(MyLab25 Gold; Esaote, Italy) and analyzed the resulting images using
quantitative measurements (mm) of the subcutaneous tissue between the anatomic
planes (dermis and muscular fascia), at the same points that we demarcated and
evaluated using the cutometer. The probe was positioned on the previously
demarcated points in the treatment area (Figure
1), with coupling gel and without tissue compression.
We took photographs with a digital camera
(Canon EOS Rebel T3i, Canon USA Inc., Melville, NY, USA) at pretreatment and at
the final follow-up, 90 days after the treatment.
To avoid bias in relation to the
effectiveness of the treatment, we applied a routine standard method for
measuring fat reduction. This involved multiple measurement modalities: waist
circumference measurements using a measuring tape, as well as fat-layer
thickness measurements using skin fold calipers and diagnostic ultrasound [21].
We took the same care with regard to the standardized evaluations of skin firmness, which we performed with a cutometer.
For the statistical analysis, we used Graph
Pad Prism 6 (La Jolla, CA, USA). We assessed the normality of the data
distribution with the Shapiro-Wilk test. According to these results, we
analyzed the differences between the pretreatment and post treatment
measurements using either a one-way analysis of variance and Tukey’s
multiple-comparisons test or the Friedman test and Dunn’s multiple-comparisons
test. The level of significance for all tests was P<0.05.
RESULTS
We treated 21 subjects, of whom 18 (85%) were female. The subjects’
mean (SD) age was 30 (7) years. The average body weight at pretreatment was
almost the same as at post treatment; the BMI slightly declined from
pretreatment to post treatment. However, there
were no statistically significant differences in pretreatment and post
treatment body
weight or BMI (Table 1).
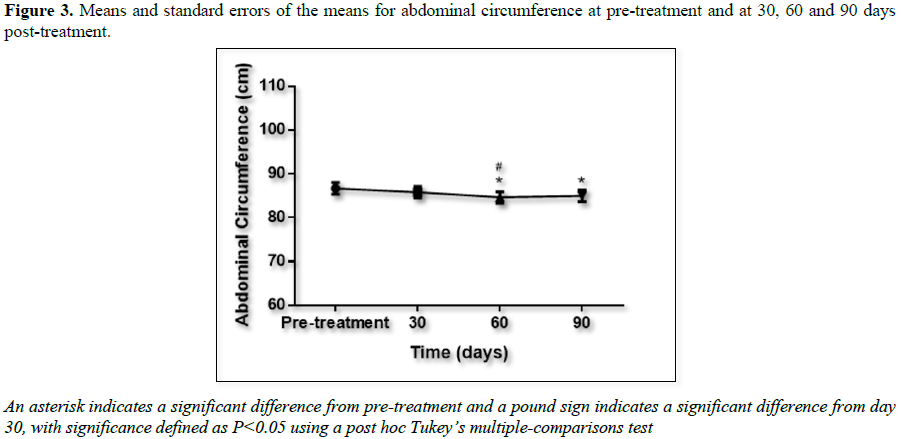
The waist circumference data are presented in
Figure 3. There were statistically
significant differences between the measurements after 60 and 90 days when
compared to pretreatment and also between the measurements after 60 days when
compared to those after 30 days.
We analyzed the skin fold-caliper data to
assess treatment efficacy. There was a statistically significant decrease in
skin fold thickness in the treated areas at all follow-up time points when
compared to the pretreatment measurements (Figure
4).
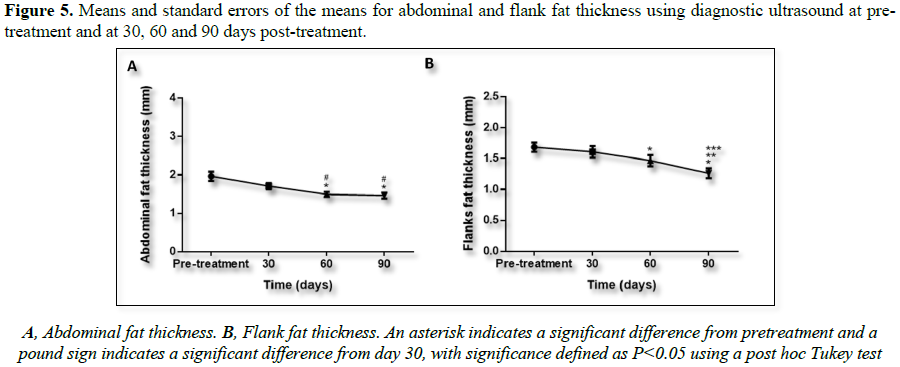
Ultrasound images were analyzed to calculate
fat layer reduction. Figure 5 shows
representative ultrasound images captured at pretreatment and at 30, 60 and 90
days after the treatment. Reduction in fat layer was statistically significant
in both treated regions: abdomen and flanks.
DISCUSSION
Cryolipolysis is frequently used for
localized fat treatment [22]. This treatment is performed with small, medium or
large vacuum-pressure applicators, which extract heat from both sides of a skin
fold and reduce blood flow by simultaneously compressing the tissue and
promoting cold-induced vasoconstriction [6]. In addition to the expected
reduction in fat-layer thickness, patients and clinicians have often observed
visible skin tightening in cryolipolysis treatment areas [15,16].
Skin is composed of various types of cells in
two layers: epidermis and dermis [23]. Subcutaneous adipose tissue is a soft
connective tissue that is located under the dermis [24]. Both the skin and the
subcutaneous adipose tissue are visco elastic and the toughness of these
tissues is determined by their density and by the arrangement of type I
collagen [25]. Skin tightening can be achieved through thermal (heat-based)
injury to the dermis using radio frequencies; intense pulsed light; fractional,
high-potency lasers; or focused ultrasound. These techniques are frequently
used in aesthetic procedures [26-31]. Heat shock proteins (HSPs)—which can be
induced using a wide variety of stressors (including heat, cold, ultraviolet
radiation, oxidative stress, ischemia, cellular-energy depletion, and
inflammation)—have a general protective function and enable cellular survival [32-34].
When collagen is heated, its heat-sensitive bonds begin to break down, turning
from an organized crystalline structure into a disorganized gel. This process
induces the synthesis of both HSP and inflammatory mediators such as tumor
necrosis factor-α, interleukin and transforming growth factor-β, which are then
released into the injured tissue [25,28,35,36]. Once these mediators are
released into the tissue, they trigger are pair cascade by activating fibroblasts
and other types of cells [33,37,38]. Researchers have shown that this type of
treatment also induces neo collagen formation, thus leading to reduced skin
flaccidity [39,40]. Cryolipolysis is an aesthetic procedure that reduces
adipose tissue through exposure to cold temperatures and it is generally
well-tolerated, with only mild side effects such as bruising, transient
neuralgia, erythema and tenderness [41]. The thermal shock of cryolipolysis
activates a repair cascade in the skin and promotes skin tightening.
Researchers have described the cutometer as
an important and effective tool for objective, noninvasive measurements of
biomechanical skin properties; it yields but absolute and relative data [18,42].
Cutometers have been widely used to evaluate human skin’s viscoelastic
properties using the suction method and cutometer-specific R0
through R9 values have been analyzed using instrument software [18].
Researchers have claimed that the R0 (Uf) parameter is
the best way to measure and quantify skin firmness or distensibility [20,43].
The purpose of this study was to evaluate both the extent to which
cryolipolysis improved skin tightness through the use of quantitative measurements and the effectiveness of this
treatment at reducing localized abdominal fat.
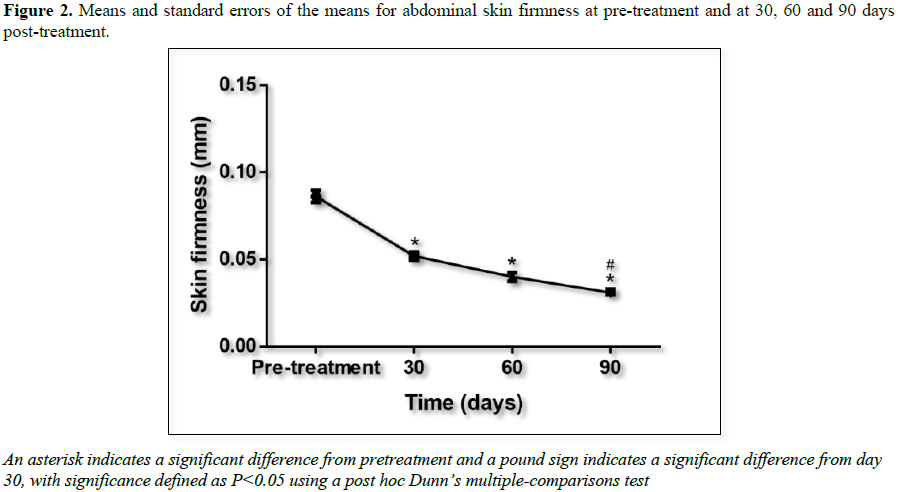
We observed that cryolipolysis treatment
significantly improved skin firmness (i.e., by producing lower values of R0 that indicate greater
firmness and lower skin distensibility). We noticed a significant difference in
this tissue property after cryolipolysis. The mechanism through which
cryolipolysis induces skin firmness is not well-understood, but it seems to
result from cold-stimulated collagen production [13]. Although Carruthers et al.
[16] suggested that the vacuum suction during cryolipolysis could stimulate neo
collagenesis via mechanical stretching of the fibroblasts, in the same study,
the authors also observed skin tightening after the use of a plate-surface
applicator, which lacks a vacuum.
Indeed, mechanical forces lead to biochemical
and molecular responses; in other words, a stretch in the cell membrane would
be transmitted via the cytoskeletal network to induce the synthesis of an
extracellular matrix from the fibroblasts [44,45]. The process of converting
physical forces into biochemical signals and then integrating these signals
into cellular responses is referred to as mechano-transduction [46].
Accordingly, when chronically stretched beyond its physiological limits, skin grows;
the increased surface area reduces the mechanical load [47]. Similarly, when
fibers are subjected to chronic excessive stretching, they may undergo
fragmentation, which results in a loss of the ability to return to their
original state; this makes the skin more plastic [23,47]. However, this skin
laxity is not observed after treatment with cryolipolysis. During this
treatment, which usually lasts around 60 min, the skin and adipose tissue
deform, cool down and suffer moderate ischemic injury [5]. The crystallization caused by
the cooling of the targeted adipocytes induces apoptosis in these cells;
although numerous researchers have reported that this cooling does not affect
tissues that are rich in water, this cold shock could be sufficient to activate
HSP and promote a subclinical inflammatory response. Supporting evidence
indicates that lipids form intracellular crystals at around 10°C, as compared
to water, which freezes at 0°C [48,49]. Consequently, thermal shock could
induce a subclinical inflammatory response and activate the fibrogenic process,
which could explain the skin tightening observed in cryolipolysis [5,16,50]. It
is important to emphasize that researchers in histologic studies have realized
that cryolipolysis treatment causes significant destruction of fat cells due to
cold, as well as substitution of the adipocytes with connective tissue, which
indicates fibroblast activation [51,52]. In this study, the cutometer
measurements indicated improved skin firmness, which may be associated with
remodeled skin collagen with improved density (Figure 2). The cutometry results suggest that this effect is
probably due to the cold stimulation of the fibroblasts.
In this study, we also used noninvasive
methods to measure the adipose-tissue thickness: calipers and ultrasound. These
results also demonstrate that non-invasive cryolipolysis is effective at
decreasing the thickness of the localized fat layer in the flanks and abdomen.
Klein et al. [53] established the safety of multiple same-day treatments (in
the abdomen and both flanks); in that study, each subject received treatment on
between one and five areas in the same day and each area received only one 60
min cooling cycle at -8°C.
In this study, the subjects did not show
significant changes in body weight (P=0.40),
as indicated in Table 1. However, the cryolipolysis treatment reduced the thickness of their
fat layers, as shown in all forms of measurement. Several researchers have
used waist-circumference measurements to determine
the efficacy of aesthetic procedure sin terms of fat reduction [13,21,54]. With
regard to waist circumference, the mean reduction was statistically significant
in this study. The other measurements also showed significant reductions in fat
(P<0.05). The calipers showed a 23.55%
reduction in the abdomen (Figure 4A) and
a 25.69% reduction in the flanks (Figure
4B). The diagnostic ultrasound showed a reduction of 25.83% in the abdomen (Figure 5A) and 19.05% in the flanks (Figure 5B). All these results are
based on a comparison of the pretreatment values and the values measured at 90
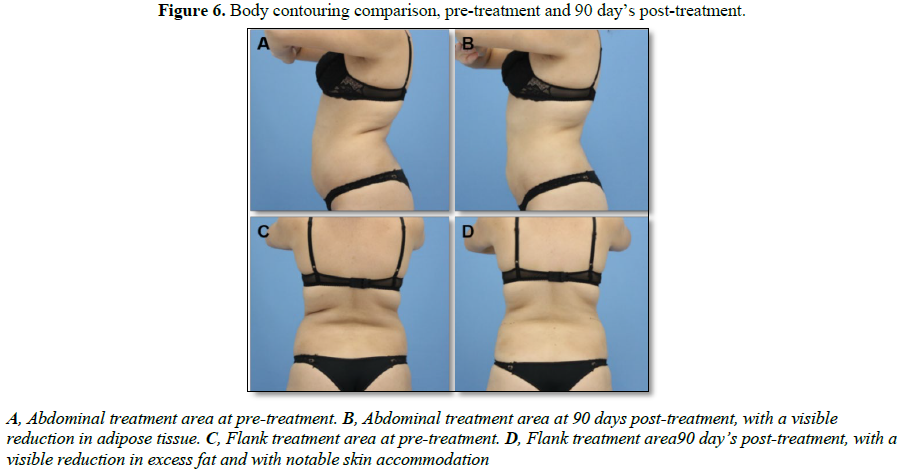
days post-treatment. Photographs of the clinical results are also shown in Figure 6.
No significant complications occurred during
the treatment. This study’s results corroborated the findings of the systematic
review conducted by Ingargiola et al. [22] regarding fat reduction based on
caliper and ultrasound measurements. In that review [22], the reductions in the
caliper measurements were 14.67% to 28.50% and the reductions in the ultrasound
measurements were 10.3% to 25.5%. Although the results from our study shown
considerable similarity to those in Ingargiola’s review study [22], this
comparison has limitations. Notably, the designs of the 19 studies differ from
that of our study in terms of treatment time, and there is insufficient
information about the temperatures used in the cooling of some studies (variable
cooling intensity factor (CIF)/value per mill watt per square centimeter (mW/cm2)).
Our clinical results suggest that rapid
cooling affects not only subcutaneous fat tissue but also skin tissue. This
action on the skin is probably due to inflammatory mediators, which initiate
the tissue-repair and regeneration pathways.
CONCLUSION
This study’s results indicate that
cryolipolysis treatment reduces fat-layer thickness and in improves skin
tightening. The exact mechanisms behind this effect remain unknown; however, in
this study, cutometer measurements demonstrated improved skin tightening in the
treated areas. This clinical investigation should encourage researchers to
complete further studies so as to better understand the mechanisms by which HSP
and/or inflammatory mediators stimulate the skin after cryolipolysis treatment.
1.
Manstein
D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, et al. (2008) Selective
cryolysis: A novel method of non-invasive fat removal. Lasers Surg Med 40:
595-604.
2.
Pinto H,
Ricart-jané D, Pardina E (2014) Pre and post lipocryolysis thermic conditioning
enhances rat adipocyte destruction. Cryo Lett 35: 154-160.
3.
Pinto HR,
Garcia-Cruz E, Melamed GE (2012) A study to evaluate the action of
lipocryolysis. Cryo Lett 33: 176-180.
4.
Pinto H,
Arredondo E, Ricart-Jane D (2013) Evaluation of adipocytic changes after a
simil-lipocryolysis stimulus. Cryo Lett 34: 100-105.
5.
Jalian
HR, Avram MM (2013) Cryolipolysis: A historical perspective and current
clinical practice. Semin Cutan Med Surg 32: 31-34.
6.
Sasaki GH,
Abelev N, Tevez-Ortiz A (2014) Non-invasive selective cryolipolysis and
reperfusion recovery for localized natural fat reduction and contouring.
Aesthetic Surg J 34: 420-431.
7.
Derrick
CD, Shridharani SM, Broyles JM (2015) The safety and efficacy of cryolipolysis:
A systematic review of available literature. Aesthetic Surg J 35: 830-836.
8.
Dierickx
CC, Mazer J-M, Sand M, Koenig S, Arigon V (2013) Safety, tolerance and patient
satisfaction with non-invasive cryolipolysis. Dermatol Surg 39: 1209-1216.
9.
Boey GE,
Wasilenchuk JL (2014) Enhanced clinical outcome with manual massage following
cryolipolysis treatment: A 4 month study of safety and efficacy. Lasers Surg
Med 46: 20-26.
10.
Bernstein
EF (2016) Long-term efficacy follow-up on two cryolipolysis case studies: 6 and
9 years post-treatment. J Cosmet Dermatol 15: 561-564.
11.
Wanitphakdeedecha
R, Sathaworawong A, Manuskiatti W (2015) The efficacy of cryolipolysis
treatment on arms and inner thighs. Lasers Med Sci 30: 2165-2169.
12.
Stevens
WG, Bachelor EP (2015) Cryolipolysis conformable-surface applicator for
non-surgical fat reduction in lateral thighs. Aesthetic Surg J 35: 66-71.
13.
Meyer PF,
Silva RMV da, Oliveira G, Tavares MA da S, Medeiros ML, et al.(2016) Effects of
cryolipolysis on abdominal adiposity. Case Rep Dermatol Med 2016: 1-7.
14.
Harrington
JL, Capizzi PJ (2017) Cryolipolysis for non-surgical reduction of fat in the
lateral chest wall post-mastectomy. Aesthetic Surg J 37: 715-722.
15.
Stevens
WG (2014) Does cryolipolysis lead to skin tightening? A first report of
cryodermadstringo. Aesthet Surg J 34: NP32-NP34.
16.
Carruthers
J, Stevens WG, Carruthers A, Humphrey S (2014) Cryolipolysis and skin
tightening. Dermatol Surg 40: S184-S189.
17.
Savacini
MB, Bueno DT, Molina ACS, Lopes ACA, Silva CNM, et al. (2018) Effectiveness and
safety of contrast cryolipolysis for subcutaneous-fat reduction. Dermatol Res
Pract 2018: 1-9.
18.
Ohshima
H, Kinoshita S, Oyobikawa M, Futagawa M, Takiwaki H, et al. (2013) Use of
cutometer area parameters in evaluating age-related changes in the skin
elasticity of the cheek. Ski Res Technol 19: 238-242.
19.
Worret
WI, Jessberger B (2004) Effectiveness of LPG? Treatment in morphea. J Eur Acad
Dermatol Venereol 18: 527-530.
20.
Chan HH,
Lam LK, Wong DS, Kono T, Trendell-Smith N (2004) Use of 1,320 nm Nd:YAG laser
for wrinkle reduction and the treatment of atrophic acne scarring in Asians.
Lasers Surg Med 34: 98-103.
21.
Auh SL,
Iyengar S, Weil A, Bolotin D, Cartee TV, et al. (2018) Quantification of
non-invasive fat reduction: A systematic review. Lasers Surg Med 50: 96-110.
22.
Ingargiola
MJ, Motakef S, Chung MT, Vasconez HC, Sasaki GH (2015) Cryolipolysis for fat
reduction and body contouring. Plast Reconstr Surg 135: 1581-1590.
23.
Silver
FH, Seehra GP, Freeman JW, DeVore D (2002) Viscoelastic properties of young and
old human dermis: A proposed molecular mechanism for elastic energy storage in
collagen and elastin. J Appl Polym Sci 86: 1978-1985.
24.
Comley K,
Fleck NA (2010) The toughness of adipose tissue: Measurements and physical
basis. J Biomech 43: 1823-1826.
25.
Arnoczky
SP, Aksan A (2001) Thermal modification of connective tissues: Basic science
considerations and clinical implications. Instr Course Lect 50: 3-11.
26.
Brightman
LA, Brauer JA, Anolik R, Weiss E, Karen J, et al.(2009) Ablative and fractional
ablative lasers. Dermatol Clin 27: 479-489.
27.
Alizadeh
Z, Halabchi F, Mazaheri R, Abolhasani M, Tabesh M (2016) Review of the
mechanisms and effects of non-invasive body contouring devices on cellulite and
subcutaneous fat. Int J Endocrinol Metab 14: e36727.
28.
Hantash
BM, Ubeid AA, Chang H, Kafi R, Renton B (2009) Bipolar fractional
radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers
Surg Med 41: 1-9.
29.
Gotkin
RH, Sarnoff DS, Cannarozzo G, Sadick NS, Alexiades-Armenakas M (2009) Ablative
skin resurfacing with a novel microablative CO2 laser. J Drugs
Dermatol 8: 138-144.
30.
Bogle MA,
Dover JS (2009) Tissue tightening technologies. Dermatol Clin 27: 491-499.
31.
Sklar LR,
El Tal AK, Kerwin LY (2014) Use of transcutaneous ultrasound for lipolysis and
skin tightening: A review. Aesthetic Plast Surg 38: 429-441.
32.
Chioran
A, Duncan S, Catalano A, Brown TJ, Ringuette MJ (2017) Collagen IV trafficking:
The inside-out and beyond story. Dev Biol 431: 124-133.
33.
Fonager
J, Beedholm R, Clark BF, Rattan SI (2002) Mild stress-induced stimulation of
heat-shock protein synthesis and improved functional ability of human
fibroblasts undergoing aging in vitro. Exp Gerontol 37: 1223-1228.
34.
Park JH,
Lee JW, Kim YC, Prausnitz MR (2008) The effect of heat on skin permeability.
Int J Pharm 359: 94-103.
35.
Reddy BY,
Hantash BM (2009) Emerging technologies in aesthetic medicine. Dermatol Clin
27: 521-527.
36.
Martella
A, Raichi M (2017) Photoepilation and skin photorejuvenation: An update.
Dermatol Rep 9: 7116.
37.
Broughton
G, Janis JE, Attinger CE (2006) The basic science of wound healing. Plast
Reconstr Surg 117: 12S-34S.
38.
Gurtner
GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration.
Nature 453: 314-321.
39.
Bjerring
P (2004) Photorejuvenation - An overview. Med Laser Appl 19: 186-195.
40.
Alster
TS, Lupton JR (2007) Non-ablative cutaneous remodeling using radiofrequency
devices. Clin Dermatol 25: 487-491.
41.
Stevens
WG, Pietrzak LK, Spring MA (2013) Broad overview of a clinical and commercial
experience with coolsculpting. Aesthetic Surg J 33: 835-846.
42.
Cua AB,
Wilhelm KP, Maibach HI (1990) Elastic properties of human skin: Relation to
age, sex and anatomical region. Arch Dermatol Res 282: 283-288.
43.
Catapani
LB, da Costa Gonçalves A, Candeloro NM, Rossi LA, de Oliveira Guirro E (2016)
Influence of therapeutic ultrasound on the biomechanical characteristics of the
skin. J Ther Ultrasound 4: 1-21.
44.
Chiquet
M, Gelman L, Lutz R, Maier S (2009) From mechanotransduction to extracellular
matrix gene expression in fibroblasts. Biochim Biophys Acta Mol Cell Res 1793:
911-920.
45.
Silver FH
(2009) The importance of collagen fibers in vertebrate biology. J Eng Fiber
Fabr 4: 9-17.
46.
Huang H,
Kamm RD, Lee RT (2004) Cell mechanics and mechanotransduction: Pathways, probes
and physiology. AJP Cell Physiol 287: C1-11.
47.
Buganza
TA, Gosain AK, Kuhl E (2012) Stretching skin: The physiological limit and
beyond. Int J Non Linear Mech 47: 938-949.
48.
Karow AM,
Webb WR (1965) Tissue freezing. Cryobiology 2: 99-108.
49.
Yiu W,
Basco MT, Aruny JE, Cheng SW, Sumpio BE (2007) Cryosurgery: A review. Int J
Angiol 16: 1-6.
50.
Mostafa
MS, Elshafey MA (2016) Cryolipolysis versus laser lipolysis on adolescent
abdominal adiposity. Lasers Surg Med 48: 365-370.
51.
Fritz K,
Salavastru C, Vanaman M, Fabi SG, Cox SE, et al. (2016) Non-invasive
subcutaneous fat reduction: A review. Dermatol Surg 42.
52.
Avram MM,
Harry RS (2009) Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg
Med 41: 703-708.
53.
Klein KB,
Bachelor EP, Becker EV, Bowes LE (2017) Multiple same day cryolipolysis
treatments for the reduction of subcutaneous fat are safe and do not affect
serum lipid levels or liver function tests. Lasers Surg Med 49: 640-644.
54.
Mahmoud
ELdesoky MT, Mohamed Abutaleb EEL, Mohamed Mousa GS (2016) Ultrasound
cavitation versus cryolipolysis for non-invasive body contouring. Australas J
Dermatol 57: 288-293.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)