766
Views & Citations10
Likes & Shares
Background: The
most common request among facial rejuvenation procedures is the augmentation of
the nasolabial fold.
Objective: To evaluate the safety, efficacy and
patient satisfaction with the use of a new cross-linked hyaluronic acid (HA)
based dermal filler (Intraline 2, Canada Inc., Kelowna BC, Canada) in reducing
medium to deep nasolabial folds.
Materials
and Methods: This was a single center, blind evaluator, 300-day
study in which 70 patients with moderate to deep, nasolabial folds were treated
at their baseline visit with up to three 1mL syringes of HA. The physician and
evaluator assessed patients 7 days after treatment and then every month after
the initial treatment for 10 months (300 days).Moreover, patient satisfaction
was measured at 1,3,6 and 10m through a self-evaluation questionnaire.
Results:
Subjects experienced statistically significant improvement in nasolabial folds
and maintained those results for more than 240 days. In proximity of the end of
the observational period (300 days) the studied area revealed minor
reabsorption of the product being at all times better than baseline. Patient
satisfaction scores were rather excellent or very good for all the length of
the study.
Conclusion:
Injectable HA new cross-linked based dermal filler (Intraline 2, Canada Inc.,
Kelowna BC, Canada) was efficacious in reducing medium to deep nasolabial folds,
resulting in satisfactory corrections up to 300 days and excellent patient
compliance and satisfaction rate.
INTRODUCTION
Dermal fillers use has grown exponentially in the last decade. Their
use extend from rejuvenating purposes, facial features enhancement, to skin
scars camouflage [1]. The key features of the treatment rely on patient
compliance and satisfaction rate [2-6]. Generally measured through satisfaction
questionnaires [7], and safety, efficacy and lasting effect of the corrections
[8].
Nasolabial folds (NLF) are natural bilateral dynamic creases that form
when zygomatic muscles contract due to facial motility. NLF’s extend from the
side of the nose to the corner of the mouth. They tend to accentuate with aging
and volume depletion, being its augmentation among the most requested
procedures in aesthetic medicine [9,10]. Several dermal fillers have been used
to treat this in esthetism [11], being hyaluronic acid dermal fillers among the
most popular due to their good safety and efficacy profile.
The use of a new cross-linked hyaluronic acid (HA) based dermal filler
(Intraline 2, Canada Inc., Kelowna BC, Canada), was tested for the treatment of
moderate to severe NLF, regarding patient satisfaction, safety, efficacy and
lasting effect.
MATERIALS AND METHODS
Eligible participants were women aged 30 and older seeking tissue
augmentation treatments for the nasolabial folds. To be qualified to receive
injections for those indications, participants had to have a wrinkle score
between 2-4 (moderate to extreme wrinkles) on the wrinkle severity scale (WSS).
The details of the scale are shown in Table
1. After local ethics committee approval, the procedure and study design
were discussed with patients and informed consents were obtained.
Exclusion criteria included poor general health, known hypersensitivity
or allergy to the treatment components, breastfeeding or pregnancy, previous
permanent fillers treatments in the area, or temporal fillers in the area in
the previous 10 months. Other exclusion criteria included; history of
autoimmune diseases; active skin disease, irritation, or inflammation in the
target areas of injection.
A new cross-linked hyaluronic acid (HA) based dermal filler (Intraline
2, Canada Inc., Kelowna BC, Canada) was used. The syringes contain 1mL of
cross-linked HA the maximum volume per patient did not exceed 3ml.
Seventy evaluable patients with moderate to severe nasolabial folds who
met all study inclusion and lack exclusion criteria were enrolled into this
single center, evaluator-masked, study.
Each subject underwent one treatment with up to three 1mL syringes of
HA. Each HA syringe was attached to a 0.5-inch, 30-G needle in preparation for
injection. The same physician treated all patients in a similar manner. The
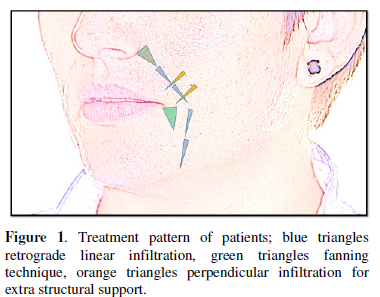
area to be treated was properly cleansed with chlorhexidine. The HA was
deposited in the superficial subcutaneous plane using a serial puncture, linear
retrograde technique; directed straight along the folds. At the corner of the
mouth (oral commissure) and in the paranasal area (alar base) a fanning
technique was use to give extra structural support. The patients were ask to
move the folds during the procedure to reveal muscular action and points of
structural breakdown. Extra material was delivering perpendicular to these
areas. The treatment design is shown in Figure
1. Any skin blebs were massaged down after administration. Total product
administered varied per patient based on the severity of the folds, with most patients
receiving an average of 2 mL (~2 syringes) per treatment session. Total volume
at the per treatment was recorded. Patients followed up 7 days after treatment
and then every 30 days after the initial treatment session for 300 days.
Self-assessment questionnaire (SAQ) were applied to patients at 1, 3, 6
and 10 months to evaluate compliance and satisfaction rate. Details of SAQ are
show in Figure 2.
Outcome and
Statistical Analysis
Standardized
photographs were taken from the front, left, and right sides of each
participant using a Nikon Camera (D-610, lens 24-85mm) at a given distance
(2,5mts) with a standardized illumination (NikonSB-700 Flash) set at each
visit. The blind observer assessed aesthetic improvement of the nasolabial
folds at each visit using the WSS which was parallel with the initial value for
that patient and baseline photographs as reference. According to the units
displaced in the scale the outcome was informed as: +2 much worse, +1 worse, 0
identical, -1 improved, -2 much improved, -3 very much improved.
Participants completed four satisfaction questionnaires at 1, 3, 6 and
10 months after the treatments. The former, assess overall satisfaction
considering the treatment area.
The questionnaire focused on the aesthetic results after treatment and
contained 7single-choice questions. For each single-choice question, a scale of
5 possible score options (scarse1, medium2, good3, very good4, excellent5), was
provided, so that participants had opportunities to provide their feedback
regarding treatment. The SAQ scores were arbitrarily defined according to their
range in: Excellent (35-29), very good (28-22), good (21-15), medium (14-8) or
scarse (7 or less).
Adverse events (AEs) were monitored throughout the study. At each study
visit, the investigators assessed erythema, edema and swelling, bruising, lumps
and bumps, pain and tenderness, and pruritus on a scale of 0 (none) to 3
(severe). During the entire duration of the study patients recorded the
possible adverse events and rate them using the same scale within the SAQ.
Statistical Analysis
Statistical analysis was done with excel 13 (windows 10). P .05 was
considered to be statistically significant, and .001 was considered to be highly
statistically significant.
RESULTS
Seventy female
Caucasian patients were enrolled in the study. The mean age of the patients was
58 (range 37-68).
Eight patients
were lost during the length of the study, sixty two patients completed the
study. The mean amount of HA injected for
the NLF was 1.8 mL, with a range from 1-3mL.
Mean baseline NLF
wrinkles according to WWS was 3.3. The severity of the folds improved by 1,4
point scale by day 7 (p < .001) and
remained statistically significantly improved by day 300 (p = .003), although by
day 180, the level of improvement had begun to decrease.
Satisfaction
questionnaires was rated as very good or excellent for the majority in the
controls at 1 (median 30,55), 3 (median 29,75), 6 months (median 28,8)and at
the last 10m control (median 26,6). The global median for all the study period
was 28,925. Details are shown on Table
2.
Side effects
included bruising 4% (n=6), swelling 3% (n=5), bumpiness 2% (n=3), asymmetry
1,6% (n=2), and erythema/discoloration 0,62% (n=1) that were primarily
self-limiting within the first 1–2 weeks postinjection. Tyndall effect,
granulomatous or nodular reactions, and focal necrosis were not registered.
DISCUSSION AND CONCLUSIONS
A successful filler treatment is defined as a good aesthetic result,
free of complications, with a good evolution in time and maximal patient
compliance and satisfaction [12-15]. The former is possible with the correct
selection of the patient, material and technique.
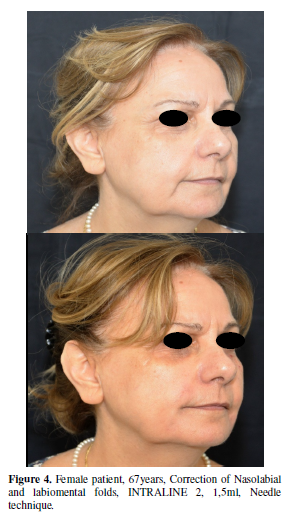
A new cross-linked HA dermal filler (Intraline 2, Canada Inc., Kelowna BC, Canada) probed to be effective in treating moderate to severe nasolabial folds with consistent results, maintained during all along study length. Patients and physician satisfaction, was very good or excellent for the majority.Particularly interesting is the fact that the severity of wrinkles improved even at day 300 and satisfaction also remained good even at 10 months. The long lasting action and patient satisfaction was probably due to the cross-linking technology of this new medical device, which is characterized by isovolumetric degradation. While hyaluronic acid reabsorbs, water molecules are able to bind the rest of the HA structure to maintain the whole structure in place, until the last of the HA molecules is reabsorbed. Moreover the filler material interacts with the recipient site cells and increases their metabolism, collagen synthesis and hydration, which may explain why the benefits of the treatment are present even after the products reabsorbs. The adverse events were few and self-limited.
REFERENCES
- Wollina U, Goldman A (2015) Fillers for
the improvement in acne scars. Clin Cosmet Investig Dermatol 8:
493-499.
- Fabi SG, Champagne JP, Nettar KD, Maas
CS, Goldman MP (2013) Efficacy and Safety of and Patient Satisfaction with
Injectable Hyaluronic Acid with 0.3% Lidocaine Hydrochloride for the
Treatment of Superficial Perioral Lines or Superficial Lateral Canthal
Lines. Dermatol Surg 39: 1613-1620.
- Rzany B, Cartier H, Kestemont P,
Trevidic P, Sattler G, et al. (2012) Full‐Face Rejuvenation Using a Range of
Hyaluronic Acid Fillers: Efficacy, Safety, and Patient Satisfaction over
6 Months. Dermatol Surg 38: 1153-1161.
- Smith L, Cockerham K (2011) Hyaluronic
acid dermal fillers: can adjunctive lidocaine improve patient satisfaction
without decreasing efficacy or duration?. Patient preference and adherence
5: 133.
- Buntrock H, Reuther T, Prager W,
Kerscher M (2013) Efficacy, Safety, and Patient Satisfaction of a
Monophasic Cohesive Polydensified Matrix Versus a Biphasic Nonanimal Stabilized
Hyaluronic Acid Filler After Single Injection in Nasolabial Folds.
Dermatol Surg 39: 1097-1105.
- Carruthers J, Carruthers A, Monheit GD,
Davis PG (2010) Multicenter, randomized, parallel-group study of
onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth,
cohesive gel) alone and in combination for lower facial rejuvenation:
satisfaction and patient-reported outcomes. Dermatol Surg 36: 2135-2145.
- Malay S, Chung K (2013) How to use
Outcome Questionnaires: Pearls and Pitfalls. ClinPlast Surg 40: 261-269.
- Few J, Cox S, Paradkar-Mitragotri D,
Murphy D (2015) A Multicenter, Single-Blind Randomized, Controlled Study
of a Volumizing Hyaluronic Acid Filler for Midface Volume Deficit:
Patient-Reported Outcomes at 2 Years. Aesthet Surg J 35: 589-599.
- Narins RS, Coleman WP, Donofrio LM, Maas
JC, Monheit G, et al (2010) Improvement in nasolabial folds with a
hyaluronic acid filler using a cohesive polydensified matrix technology:
results from an 18-month openlabel extension trial. Dermatol Surg 36:
1800-1808.
- Sood V, Nanda S (2012) Patient
Satisfaction with Hyaluronic Acid Fillers for Improvement of the
Nasolabial Folds in Type IV & V Skin. J Maxillofac Oral Surg 11:
78-81.
- 14. Moers-Carpi M, Sherwood S (2013)
Polycaprolactone for the Correction of Nasolabial Folds: A 24-Month,
Prospective, Randomized, Controlled Clinical Trial. Dermatol Surg 39:
457-463.
- Vedamurthy M, Vedamurthy A (2008) Dermal
Fillers: Tips to Achieve Successful Outcomes. J Cutan Aesthet Surg 1:
64–67.
- McCracken MS, Khan JA, Wulc AE, et al.
(2006) Hyaluronic acid gel (Restylane) filler for facial rhytids: lessons
learned from American Society of Ophthalmic Plastic and Reconstructive
Surgery member treatment of 286 patients. Ophthal Plast Reconstr Surg 22:
188-191.
- Muhn C, Rosen N, Solish N, Bertucci V,
et al. (2012) The evolving role of hyaluronic acid fillers for facial
volume restoration and contouring: a Canadian overview. Clin Cosmet
Investig Dermatol 5: 147-158.
- Allemann I, Baumann L (2008) Hyaluronic
acid gel (Juvéderm™) preparations in the treatment of facial wrinkles and
folds. Clin Interv Aging 3: 629-634.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Alcoholism Clinical Research
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Spine Diseases