Review Article
The Histologic Spectrum of Lupus Miliaris Disseminatus Faciei as Demonstrated By a Solitary Individual
| Lisa Mask-Bull*, Michelle B. Tarbox and Cloyce Stetson |
|
| Corresponding Author: Lisa Mask-Bull M.D., Resident of Dermatology at Texas Tech University Health Sciences Center, Department of Dermatology, 3601 4th St A100, Lubbock, Texas 79430, (806) 744-4428, Lisa.Mask@ttuhsc.edu |
|
|
Received: October 17, 2015;
Revised: November 30, 2015;
Accepted: November 9, 2015
|
|
| Citation: Mask-Bull L, Tarbox M & Stetson C (2015) The Histologic Spectrum of Lupus Miliaris Disseminatus Faciei as Demonstrated By a Solitary Individual. Dermatol Clin Res, 1(3): 57-62. |
|
| Copyrights: ©2015 Mask-Bull L, Tarbox M & Stetson C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
|
|
|
|
|
Share :
|
2431
Views & Citations1431
Likes & Shares
Lupus miliaris
disseminatus faciei (LMDF) is an uncommonly encountered and incompletely
understood acute granulomatous cutaneous eruption. Varying clinical and
histopathologic manifestations have led to differing opinions regarding the
pathogenesis and appropriate classification of this entity. Histopathologic
examination is necessary for diagnosis, usually requiring the presence of
epithelioid granulomas with caseation necrosis, as demonstrated by fully
developed lesions. However LMDF is now generally understood to include a
spectrum of histologic stages, which vary with clinical stage. We will discuss
our encounter with a 60-year-old white female who demonstrated histopathologic
features consistent with multiple stages of LMDF. Microscopically this patient
additionally showed periapocrine granulomatous inflammation. As an atypical
clinical presentation was also seen, we feel this one patient effectively
demonstrates the clinical and histopathologic heterogeneity that may be seen in
this condition. Lastly we will comment on our recognition of a questionable
correlation linking androgen stimulation of the pilosebaceous apocrine unit to
the development of LMDF.
The pathogenesis of LMDF has been met with
reasonable debate. A large number of ideas incompletely explain the
pathogenesis of LMDF, leading many to suggest that multiple factors may
actually be responsible [1]. Suggested by nomenclature, LMDF was historically
regarded as a tuberculid but this concept was refuted in 1997 due to consistent
lack of PCR or culture evidence of Mycobacterium tuberculosis [1-4]. Another proposal is that LMDF
may be a reaction to an unknown infectious agent associated with cell-mediated
immunity [1,5]. Recently, an association between Propionibacterium acnes
and LMDF has been proposed due to PCR demonstration of P. acnes in 9
cases [6]. Other theories focus on the pilosebaceous unit as central to the
pathogenesis of LMDF, since a perifollicular infiltrate is present in many, but
not all, cases of LMDF [5,7]. A primary immune response to pilosebaceous units
or follicular trauma leading to antigen exposure and subsequent granuloma
formation may be involved in the pathogenesis. The presence of a lymphocytic
perifollicular infiltrate with invasion into the follicular wall in early
lesions of LMDF supports that the initial triggering event may be lymphocyte
mediated [1,5].
Damage of the follicular wall subsequently leads to release of
follicular contents into the dermis and a granulomatous reaction then ensues
[1,5,7]. While perifollicular granulomas could represent a non-immunologic
foreign body reaction to follicular contents, evidence suggests a role for
cell-mediated immunity as supported by the presence of intense staining of
lysozyme in the epithelioid histiocytes and multinucleated giant cells [1].
Many regard LMDF to be a variant of granulomatous rosacea; however others
emphasize a distinction between these two entities, which is supported by a
clinical course of LMDF that is different than granulomatous rosacea [1,2,4,7].
It has been suggested that designation of LMDF may be most appropriate in cases
that are clinically like sarcoidosis but histologically similar to
granulomatous rosacea [8]. This places LMDF on a spectrum of granulomatous
conditions, some of which are also incompletely understood.
Case Presentation
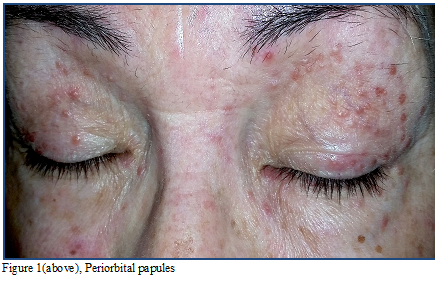
We encountered a 60-year-old female with a
6-month history of multiple mildly pruritic flesh colored periorbital papules (Figure 1). She endorsed occasional
application of hydrocortisone 1% cream but denied use of additional products.
She lacked significant past medical history, review of systems was
noncontributory and she denied usage of antiperspirants containing
aluminum-zirconium. Punch biopsy of a periorbital papule showed a discrete
perifollicular epithelioid granuloma with a central zone of caseation necrosis
(Figure 2, 3). AFB and fungal stains
were negative and examination under polarized light identified no foreign
material. Following a negative QuantiFERON-TB Gold test, a diagnosis of LMDF
was applied. The patient was started on tacrolimus 0.1% ointment BID. At
subsequent follow up 3 months later, significant improvement of the periorbital
papules was demonstrated (many leaving oval violaceous macules) but multiple
new pink papules of the bilateral axillary vaults had developed over the last
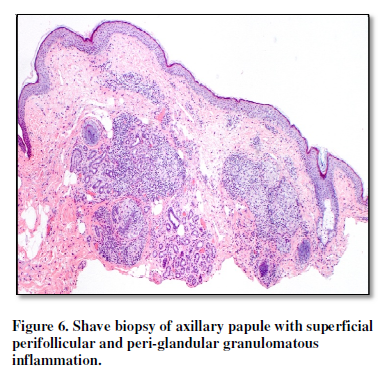
month (Figure 4). Biopsy of an
axillary papule demonstrated a mild superficial perivascular and superficial
perifollicular infiltrate composed of lymphocytes, histiocytes and neutrophils
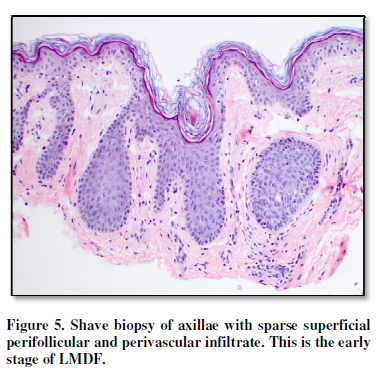
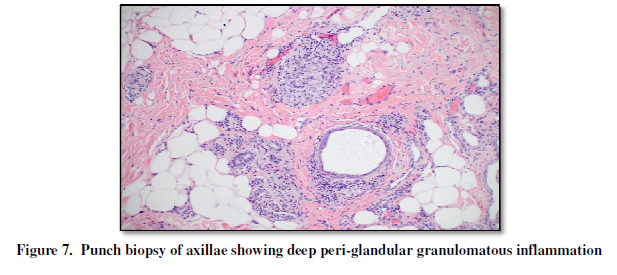
(Figure 5). In the deep dermis, a
caseating granuloma was present in addition to periapocrine granulomas (Figure 6, 7). The patient was started
on Doxycycline 100mg PO BID. A 6-month follow-up appointment confirmed only
mild improvement of the axillary lesions.
Discussion
Our patient demonstrates the varying histologic
presentations of LMDF, which have been previously well described to vary with
the stage of disease evolution at the time of biopsy [3]. Recent attention has
been devoted to this broad spectrum of histologic findings that may be
demonstrated in LMDF. This spectrum is divided into three histologic stages:
early, fully developed and late [1]. Fully developed lesions may be
additionally divided into four groups, defined by the type of granulomatous
reaction that is present [1]. Demonstrated by microscopic examination of
axillary skin of our patient, the early stage of LMDF exhibits a superficial
perivascular and perifollicular infiltrate composed predominantly of
lymphocytes and with a lesser degree of histiocytes and neutrophils (Figure 5) [1,2]. Present in fully
developed lesions and demonstrated by punch biopsy of the periorbital papule of
our patient, the characteristic histopathologic finding in LMDF is a lesion in
the superficial to mid-dermis with epithelioid cell granulomas surrounding
areas of caseation necrosis (Figure
4a,4b) [1,2]. Fully developed lesions may be associated with a ruptured
hair follicle and demonstrate superficial perifollicular granulomatous
inflammation composed of lymphocytes, histiocytes and multinucleated giant
cells surrounding caseation necrosis [1-3]. These granulomas are composed of
histiocytes, multinucleated giant cells (Langerhans or foreign body type),
scarce neutrophils and a peripheral rim of lymphocytes [1,2].
Finally, late stage lesions show perifollicular fibrosis with few scattered
histiocytes, lymphocytes and neutrophils. While recognition of the
histopathologic variability may be important in this condition, confusion is
best avoided by restricting the diagnosis of LMDF to lesions demonstrating
epithelioid granulomas with caseation necrosis [1]. As opposed to the
histopathologic variability seen in this condition, the clinical
characteristics of LMDF are generally reproducible. The typical course is one
of acute onset with spontaneous resolution over 2-4 years, usually leaving
residual pitted scars [2,4,5,9]. LMDF is predominantly a condition of young to
middle-aged individuals [5]. The appearance in individuals in the 7th
decade of life, as in our patient, is unusual. To our knowledge, the oldest
patient reported to have LMDF was 71 and few patients over the age of 50 have
been published [10,11]. Interestingly, the literature reflects a striking
gender disparity in LMDF. A recent review of 35 patients with LMDF showed that
all patients with LMDF over the age of 30 were female, whereas patients under
the age of 30 were predominantly male [11]. To our knowledge, there have been
no attempts to explain the impressive gender predilection that varies with age
in LMDF. LMDF almost always involves the face, with striking predilection for
the periorbital skin, especially the lower eyelids [2,4,12,13]. Extrafacial
involvement is uncommon, with only three prior reports of axillary LMDF
[4,2,14]. Of the three reported cases of axillary LMDF, two also occurred in
females over the age of 50 (ages 53, 55) [14,15]. The third case of axillary
LMDF occurred in a 36-year-old male [14]. If including our patient, three of
the four cases of axillary LMDF developed in females over 50 years of age; a
situation which is only of mention due to a well recognized predilection of
LMDF for an (even) younger population. As explained, the pilosebaceous
apparatus is thought to be central to the development of LMDF. Anatomic sites
of predilection of LMDF include those that are rich in pilosebaceous units, but
lesions of LMDF also occur in regions of skin that contain apocrine glands such
as the periocular skin, the axillae and genitalia [9]. The
distribution of skin lesions in our patient strictly and symmetrically involved
the periocular and axillary skin. Biopsies demonstrated both perifollicular and
periapocrine granulomatous inflammation. While the latter could simply have
developed due to adjacent inflammation, it could also represent apocrine
involvement in this patient’s disease. And because apocrine and pilosebaceous
glands are intimately associated with hair follicles, it seems possible that
all could be involved in some cases of LMDF [16,17].
As both apocrine and
sebaceous glands are under the control of androgens, we wonder if such hormonal
interactions could explain some of the disparities we have mentioned above: the
general predilection of LMDF for a young age group, the male predominance under
30 years of age, the striking female predominance in females over 30, and the
four cases of axillary LMDF appearing in ideal candidates for either androgen
excess or hyperreactivity [17,18]. A similar conclusion was posed by Walchner
et al following their experience with a 30-year-old female who initially
developed LMDF during the third trimester of pregnancy [19]. LMDF initially
improved in the postpartum period but subsequently flared 6 months postpartum
[19]. Interestingly, the patient later developed cutaneous lupus erythematosus.
The authors concluded that a possible common pathogenic pathway between LMDF
and LE might exist, initially involving a localized autoimmune-like process
restricted to sebaceous glands with subsequent antigen expression provoked by
hormonal factors [19]. Our patient presented with LMDF at a late age and in an
atypical cutaneous location, whilst simultaneously demonstrating multiple
stages of LMDF histopathologically. An extensive review of the literature led
to recognition of notable trends in LMDF. This information was applied to our
patient’s case, facilitating conclusions that support the relevance of
pilosebaceous units in the pathogenesis of LMDF.
- Echols K, Fang F, Patterson
JW (2014) A Review of Lupus Miliaris Disseminatus Faciei-Like
Histopathologic Changes in 10 Cases. J Clin Exp Dermatol Res 5: 1-5.
- Khokhar O, Khachemoune A
(2004) A case of granulomatous rosacea: Sorting granulomatous rosacea from
other granulomatous diseases that affect the face. Dermatol Online J 10:
6.
- Micaels J, Cook-Norris RH,
Lehman JS, Gibson LE (2014) Adult with papular eruption on the central
aspect of the face. J Amer Acad Dermatol 71: 410-412.
- Dae SK, Lee KY, Shin JY, Roh
MR, Lee MG (2008) Lupus Miliaris Disseminatus Faciei Without Facial
Involvement. Acta Derm Venereol 88: 504-505.
- Rocas D, Kanitakis J (2013)
Lupus miliaris disseminatus faciei: Report of a new case and brief
literature review. Dermatol Online J 19: 4.
- Nishimoto J, Amano M,
Setoyama M (2015) The detection of Propionibacterium acnes
signatures in granulomas of lupus miliaris disseminatus faciei. J Dermatol
42: 418-421.
- Shitara A (1982)
Clinicopathological and immunological studies of lupus miliaris
disseminatus faciei. J Dermatol 9: 383–395.
- Sehgal V, Srivastaca G,
Aggarawal A, Reddy V, Sharma S (2005) Lupus Miliaris Disseminatus Faciei
Part I: Significance of Histopathologic Undertones in Diagnosis. SKINmed:
Dermatology for the Clinician 4: 151-156.
- Ming JH, Friedman PM,
Kimyai-Asadi A, Friedman ES, Hymes SR, et al. (2005) Lupus Miliaris
Disseminatus Faciei Treatment With the 1450-nm Diode Laser. Arch Dermatol
141: 143-145.
- Dekio S, Jidoi J, Imaoka C
(1991) Lupus miliaris disseminatus faciei- report of a case in an elderly
woman. Clin Exp Dermatol 16: 295-296.
- Dianawaty A, Sumiyuki M,
Fujimura T, Katsuoka K (2011) Clinical evaluation of 35 cases of lupus
miliaris disseminatus faciei. J Dermatol 38: 618-620.
- Hillen U, Schroter S,
Denisjuk N, Jansen T, Grabbe S (2006) Axillary acne agminata (lupus
miliaris disseminates faciei with axillary involvement). J Ger Soc
Dermatol 4: 858-860.
- Sehgal V, Srivastaca G,
Aggarawal A, Reddy V, Sharma S (2005) Lupus Miliaris Disseminatus Faciei
part II: A Review. SKINmed: Dermatology for the Clinician 4: 234-238.
- Bedlow AJ, Otter M, Marsden
RA (1998) Axillary acne agminata (lupus miliaris disseminates faciei).
Clin Exp Dermatol 23: 125-128.
- Farrar CW, Bell HK, Dobson
CM, Sharpe GR (2003) Facial and axillary acne agminata. Brit J Dermatol
149: 1076.
- Woollard HH (1930) The Cutaneous
Glands of Man. J Anat 64: 415-421.
- Zouboulis ChC, Degitz K
(2004) Androgen action on human skin – from basic research to clinical
significance. Exp Dermatol 13: 5-10.
- Mortimer PS, Dawber RPR,
Gales MA, Moore RA (1986) Mediation of Hidradenitis Suppurativa by
Androgens. Brit Med J 292: 245-248.
- Walchner M, Plewig G, Messer G (1998) Lupus
miliaris disseminatus faciei evoked during pregnancy in a patient with
cutaneous lupus erythematosus. Int J Dermatol 37: 864-867.