2028
Views & Citations1028
Likes & Shares
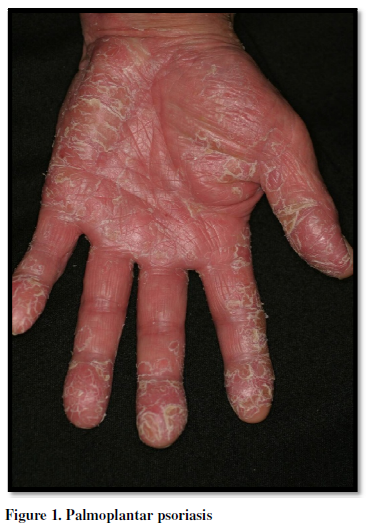
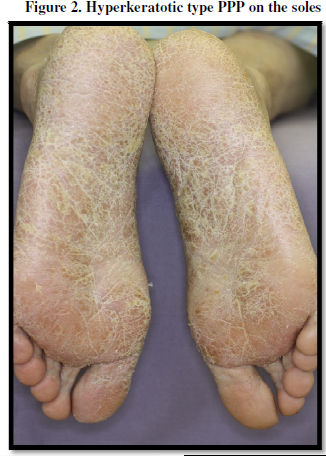
Palmoplantar pustulosis (PPP) is characterized by many
aseptic small pustules, scales, crusts and erythemas, involving the palms and
soles. PPP is frequently seen in Japan, and thus considered as a distinct
entity. By contrast, in other countries, PPP is sometimes regarded as an acral
variant of pustular psoriasis. Typical clinical features definitely differ
between PPP and plaque-type psoriasis, however, in rare cases, PPP presents
with features resembling psoriasis vulgaris or pustular psoriasis with
palmoplantar pustulation. Although extra-palmoplantar lesions of PPP are not
psoriasis, patients with PPP rarely show typical psoriasis during the course. On
the other hand, patients with generalized pustular psoriasis (GPP) can exhibit
pustular lesions on the palms and soles. Those findings strongly suggest a
close relationship between PPP and psoriasis, however, there are a number of
differences between PPP and pustular psoriasis. In this review,
clinicopathological aspects of PPP are described, and the difference from
pustular psoriasis is discussed.
INTRODUCTION
Palmoplantar pustulosis (PPP) is
characterized by aseptic small pustules, scales, crusts and erythemas on the
palms and soles. The frequency of this disease varies among different
countries, and frequently observed in Japan. PPP is sometimes regarded as a
localized variant of pustular psoriasis, while others consider PPP to be a
distinct nosological entity, different from psoriasis [1-3]. The controversial
viewpoints may depend on the different frequency of PPP. Undoubtedly, PPP is
closely related to psoriasis, and both disorders share common pathogenesis in a
number of aspects. The author has been standing a position that PPP is a
distinct entity different from pustular psoriasis. In this review, a close
relationship and differences between PPP and pustular psoriasis have been
discussed.
SIMILARITIES OF
PALMOPLANTAR PUSTULOSIS AND PSORIASIS
PPP has a predilection for females, and involves middle-aged women,
usually occurring at the age between 30-40 years. In the majority of patients,
bilateral palmar lesions antecede the plantar involvement with a few months
duration. On the other hand, only palmar involvement is occasionally seen,
while incidence of only sole involvement is much lower. Patients, especially
women patients, are either current or previous smokers, and nicotine included
in tobacco is suggested to play a role. PPP lesions are typically confined to
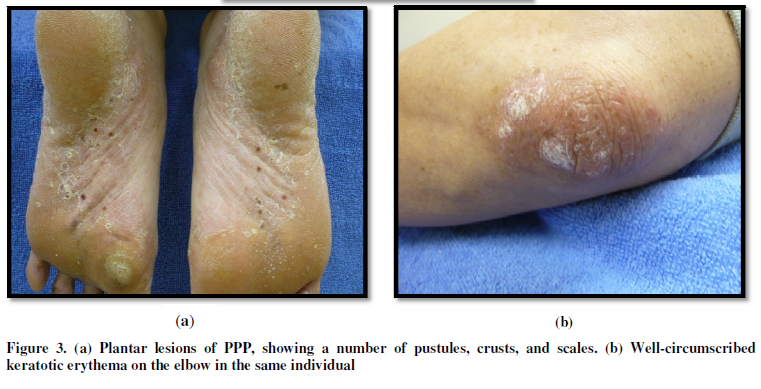
the palms and soles, while a number of erythematous lesions with scaling
sometimes appear on the trunk and/or extremities. Those extra-palmoplantar
lesions are seen either chronically or suddenly accompanied by joint pain,
following focal infections such as tonsillitis, dental infections, and
sinusitis [4]. In the cases of acute onset, solitary pustules may also be seen
on the trunk, however, severe cases mimicking pustular psoriasis are also seen.
In general, extra-palmoplantar lesions are seen more frequently in patients
with severe PPP. Compared with psoriasis, infiltration of the erythema is mild
and the lesions are neither well demarcatednor accompanied by thickened scales.
Nail lesions are frequently seen in PPP, such as subungual pustule,
hyperkeratosis, dystrophy, onycholysis, when inflammation involves the nail
matrix.
PATHOPHYSIOLOGY OF
PALMOPLANTAR PUSTULOSIS
Serum levels of IL-17 and IL-22 are increased in patients with PPP [9].
IL-23 expression is upregulated in the lesional skin of PPP [10],and IL-17 is
detected close to or in the acrosyringium [11]. Acrosyringium is reported to be
the primary target for inflammation in PPP [12, 13]. Acetylcholine is the main
inducer of sweating, and many components for cholinergic signaling have been
found in the skin. Nicotine acts on nicotinic acetylcholine receptor AChRs
(nAChRs) as an agonist, which then leads to the provocation of many functions. In
PPP lesional skin, altered nAChR expression was observed, and epidermal α7nAChR
expression was abolished compared with normal skin [14]. Patients with PPP may
be incapable of activating the endogenous nicotinic anti-inflammatory pathway,
due to a decrease of α7nAChR, and show abnormal response to nicotine.
TRIGGERING FACTORS
PPP is frequently associated with foci of chronic bacterial infection
[15]. Bacterial products stimulate enhanced production of IL-23, which triggers
T-cells to produce IL-17. Tonsillitis is the most closely associated focal
infection with PPP, and tonsillectomy improves and even completely releases
cutaneous as well as skeletal lesions [16]. In addition, odontogenic infection,
sinusitis, cholecystitis, and appendicitis also sometimes precede the onset of
PPP. These facts strongly suggest a key triggering role of bacterial infection
leading to a sequential event inducing PPP. In
vitro, bacterial infection activates tonsillar T-cells to enhance cutaneous
lymphocyte-associated antigen (CLA) expression [17] and enhances cytokine
production such as IL-6, TNF-α, and interferon-γ (IFN-γ) [18]. Toll-like
receptors (TLRs) play important roles in the innate immune responses following
bacterial infection. Heat shock proteins (HSPs) are recognized by γδ T-cell
receptors and TLR-2 and -4 [19], and may act as an endogenous and/or exogenous
signal to trigger immune responses. TLRs signal the presence of an infection
and direct the adaptive immune response against microbial antigens by inducing
proinflammatory cytokines and upregulating costimulatory molecules of antigen
presenting cells.
CO-EXISTENCE OF
PALMOPLANTAR PUSTULOSIS AND PSORIASIS
GENERALIZED PUSTULAR
PSORIASIS
Generalized pustular psoriasis (GPP) is a rare systemic disease
characterized by widespread, superficial sterile pustules over the trunk, which
often rapidly develop into
erythroderma. Pustular psoriasis is divided into generalized and
localized, and the former type includes Zumbusch type, impetigo herpetiformis
(acute GPP of pregnancy), annular and circinate form, juvenile and infantile
pustular psoriasis, and generalized form of acrodermatitis continua
(Hallopeau). PPP was previously known as ‘pustular psoriasis of the
extremities’. Baker and Ryan [21] formerly classified GPP into several
subgroups including the "palm-sole" type. Both PPP and GPP are
characterized by aseptic pustular formation, which reflect enhanced activity of
neutrophil recruitment to the skin. The pustular lesions present not as
solitary pustules, but as coalescent sheet-like pustular formations. These
facts may suggest that PPP is the palm-sole type of GPP. Clinical appearances
between both diseases are different, however, patients with GPP sometimes
present with severe pustular lesions on the palms and/or soles [4]. Infantile
GPP is seen, although rare, whereas there are no pediatric cases of PPP.
CO-MORBIDITIES OF
PALMOPLANTAR PUSTULOSIS AND GENERALIZED PUSTULAR PSORIASIS
Recently, the co-morbidities associated with PPP have been surveyed [33].
However, in this report, the representative co-morbidities with psoriasis, such
as cardiovascular diseases including ischaemic heart disease, hypertention, and
dyslipidaemia, were presented in a high ratio. Furthermore, they described that
psoriatic artyhropathy was present in 12%. It is highly suspected that they
enrolled many patients with either palmoplantar psoriasis or palmoplantar
pustular psoriasis in their study. Whether the examined patients are truly PPP,
not psoriasis, may be doubtful.
On the other hand, because GPP is a severe form of psoriasis, many
conditions such as arthralgia, metabolic syndrome, cardiovascular event,
ophthalmological involvement, and inflammatory bowel diseases are accompanied. In
addition, cholestasis frequently occurs in patients with GPP, and liver biopsy
revealed neutrophilic cholangitis [34], which suggest a pathogenic role of
activated neutrophils for liver damages. So far, pulmonary involvement in the
course of psoriasis was estimated to be extremely rare, and only a few cases of
acute respiratory distress syndrome were reported in association with GPP
and/or psoriatic erythroderma [35, 36]. Possible aetiologies such as microbial
infection, drug-induced reaction and capillary leak syndrome, via
proinflammatory cytokines such as TNF-α, IL-6, and IL-8, have been proposed.
CONCLUSION
The comparison of PPP and GPP is shown in Table 1. Although there are
many features of clinical symptoms including nail abnormality and joint
involvement in common, several different aspects should also be recognized. Recently,
psoriasis is regarded as a systemic inflammatory disorder, and various organ
involvements are seen in patients with severe psoriasis, especially with GPP,
i.e. cardiovascular disease, inflammatory bowel disease, joint manifestations,
uveitis, acute respiratory distress syndrome, and chronic kidney disease. By
contrast, organ involvement in association with PPP still needs further
investigations. Although PPP sometimes shows features overlapping with either
psoriasis or GPP, and both PPP and GPP are included in autoinflammatory
pustular neutrophilic diseases [37], PPP nevertheless should be regarded as a
distinct entity, different from acral variant of GPP.
CONFLICT OF
INTERESTS
1
Brunasso AMG, Massone C
(2010) Can we really separate palmoplantar pustulosis from psoriasis? J Eur Acad
Dermatol Venereol 24: 619-621.
2
Yamamoto T (2010) Can we
really separate palmoplantar pustulosis from psoriasis? Reply. J Eur Acad Dermatol Venereol 24: 621.
3
de Waal AC, van de
Kerkhof PC (2011) Pustulosispalmoplantaris is a disease distinct from
psoriasis. J Dermatolog Treat 22: 102-105.
4
Yamamoto T (2009) Extra-palmoplantar
lesions associated with palmoplantar pustulosis. J Eur Acad Dermatol Venereol 23:
1227-1232.
5
Andoh A, Ogawa A,
Kitamura K, Inatomi O, Fujino S, et al. (2004) Suppression of interleukin-1β-
and tumor necrosis factor-a-induced inflammatory responses by leukocytapheresis therapy in
patients with ulcerative colitis. J Gastroenterol 39: 1150-1157.
6
Laan M, Cui ZH, Hoshino
H, Lötvall J, Sjöstrand M, et al. (1999) Neutrophil recruitment by human IL-17
via C-X-C chemokine release in the airways. J Immunol 162: 2347-2352.
7
Roussel L, Houle F, Chan
C, et al. (2010) IL-17 promotes p38 MAPK-dependent endothelial activation
enhancing neutrophil recruitment to sites of inflammation. J Immunol 184:
4531-4537.
8
Griffin GK, Newton G,
Tarrio ML, et al. (2012) IL-17 and TNF-a sustain neutrophil recruitment during inflammation
through synergistic effects on endothelial activation. J Immunol 188:
6287-6299.
9
Murakami M, Hagforsen E,
Morhenn V, Ishida-Yamamoto A, Iizuka H (2011) Patients with palmoplantar
pustulosis have increased IL-17 and IL-22 levels both in the lesion and serum. Exp
Dermatol 20: 845-847.
10
Lillis JV, Guo C-S, Lee
JJ, Blauvelt A (2010) Increased IL-23 expression in palmoplantar psoriasis and
hyperkeratotic hand dermatitis. Arch Dermatol 146: 918-919.
11
Hagforsen E, Hedstrand H,
Nyberg F, Michaëlsson G (2010) Novel findings of Langerhans cells and
interleukin-17 expression in relation to the acrosyringium and pustule in
palmoplantar pustulosis. Br J Dermatol 163: 572-579.
12
Eriksson MO, Hagforsen E,
Lundin IP, Michaëlsson G (1998) Palmoplantar pustulosis: a clinical and
immunohistological study. Br J Dermatol 138: 390-398.
13
Murakami M, Ohtake T,
Horibe Y, Ishida-Yamamoto A, Morhenn VB, et al. (2010) Acrosyringium is the
main site of the vesicle/pustule formation in palmoplantar pustulosis. J Invest
Dermatol 130: 2010-2016.
14
Hagforsen E, Edvinsson M,
Nordlind K, Michaëlsson G (2002) Expression of nicotinic receptors in the skin
of patients with palmoplantar pustulosis. Br J Dermatol 146: 383-391.
15
Yamamoto T (2013) Pustuloticarthro-osteitis
associated with palmoplantar pustulosis. J Dermatol 40: 857-863.
16
Nozawa H, Kishibe K,
Takahara M, Harabuchi Y (2005) Expression of cutaneous lymphocyte-associated
antigen (CLA) in tonsillar T-cells and its induction by in vitro stimulation
with alpha-streptococci in patients with pustulosispalmaris et plantaris (PPP).
Clin Immunol 116: 42-53.
17
Yamamoto T (2011) Triggering
role of focal infection in the induction of extra-palmoplantar lesions and
pustuloticarthro-osteitis associated with palmoplantar pustulosis.
AdvOto-Rhinolaryngol 72: 89-92.
18
Murakata H, Harabuchi Y,
Kataura A (1999) Increased interleukin-6, interferon-gamma and tumor necrosis
factor-alpha production by tonsillar mononuclear cells stimulated with
alpha-streptococci in patients with pustulosispalmaris et plantaris. Acta
Otolaryngol 119: 384-391.
19
Tsan M-F, Gao B (2004) Heat
shock protein and innate immunity. Cell Mol Immunol 1: 274-279.
20
Ammoury A, Sayed FE,
Dhaybi R, Bazex J (2008) Palmoplantar pustulosis should not be considered as a
variant of psoriasis. J Eur Acad Dermatol Venereol 22: 392-393.
21
Baker H, Ryan HJ (1968) Generalized
pustular psoriasis: A clinical and epidemiological study of 104 cases. Br J
Dermatol 80: 771-793.
22
Yamamoto T (2014) Generalized
pustular psoriasis: a new emerging concept relating to autoinflammatory
disease. Autoimmun Dis Ther Approach 1: e103.
23
Tortola L, Rosenwald E,
Abel B, Blumberg H, Schäfer M, et al. (2012) Psoriasiform dermatitis is driven
by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest 122: 3965-3976.
24
Marrakchi S, Guigue P, Renshaw
BR, Puel A, Pei XY, et al. (2011) Interleukin-36-receptor antagonist deficiency
and generalized pustular psoriasis N Engl J Med 365: 620-628.
25
Onoufriadis A, Simpson
MA, Pink AE, Di Meglio P, Smith CH, et al. (2011) Mutations in IL-36RN/IL1F5
are associated with the severe episodic inflammatory skin disease known as
generalized pustular psoriasis.Am J Hum Genet 89: 432-437.
26
Farooq M, Nakai H,
Fujimoto A, Fujikawa H, Matsuyama A, et al. (2013) Mutation analysis of the
IL36RN gene in 14 Japanese patients with generalized pustular psoriasis. Hum
Mutat 34: 176-183.
27
Sugiura K, Takemoto A,
Yamaguchi M, Takahashi H, Shoda Y, et al. (2013) The majority of generalized
pustular psoriasis without psoriasis vulgaris is caused by deficiency of
interleukin-36 receptor antagonist. J Invest Dermatol 133: 2514-2521.
28
Kanazawa N, Nakamura T,
Mikita N, Furukawa F. (2013) Novel IL36RN mutation in a Japanese case of early
onset generalized pustular psoriasis. J Dermatol 40: 749-751.
29
Setta-Kaffetzi N,
Navarini AA, Patel VM, Pullabhatla V, Pink AE, et al. (2013) Rare pathogenic
variants in IL36RNunderlie a spectrum of psoriasis-associated pustulat
phenotypes. J Invest Dermatol 133: 1366-1369.
30
Kingo K, Mӧssner R, Kõks
S, Rätsep R, Krüger U, et al. (2007) Association analysis of IL19, IL20 and
IL24 genes in palmoplantar pustulosis. Br J Dermatol 156: 646-652.
31
Asumalahti K, Ameen M,
Suomela S, Hagforsen E, Michaëlsson G, et al. (2003) Genetic analysis ofPSORS1
distinguishes guttata psoriasis and palmoplantar pustulosis. J Invest Dermatol 120:
627-632.
32
Mössner R, Kingo K,
Kleensang A, Krüger U, König IR, et al. (2005) Association of TNF-238 and -308
promoter polymorphisms with psoriasis vulgaris and psoriatic arthritis but not
with pustulosispalmoplantaris. J Invest Dermatol 124: 282-284.
33
Becher G, Jamieson L,
Leman J (2014) Palmoplantar pustulosis: a retrospective review of comorbid
conditions. J Eur Acad Dermatol Venereol 2014 [Epub ahead of print].
34
Viguier M, Allez M,
Zagdanski AM, Bertheau P, de Kerviler E, et al. (2004) High frequency of
cholestasis in generalized pustular psoriasis: evidence for neutrophilic
involvement of the biliary tract. Hepatology 40: 452-458.
35
Sadeh JS, Rudikoff D,
Gordon ML, Bowden J, Goldman BD, et al. (1997) Pustular and erythrodermic
psoriasis complicated by acute respiratory distress syndrome. Arch Dermatol
133: 747-750.
36
Abou-Samra T, Constantin
JM, Amarger S, Mansard S, Souteyrand P, et al. (2004) Generalized pustular
psoriasis complicated by acute respiratory distress syndrome. Br J Dermatol 150:
353-356.
37 Naik HB, Cowen EW (2013) Autoinflammatory
pustular neutrophilic diseases. Dermatol Clin 31: 405-425.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)