755
Views & Citations10
Likes & Shares
INTRODUCTION
Aging is an essential biological process of living
organisms and it space differs markedly among different species and even among
individuals of the same species [1]. Changes in human skin due to aging are a
major concern for both the pharmaceutical and the cosmetic sectors worldwide. A
considerable amount of expenses and investments are required for the
pharmaceuticals and cosmetics intended to delay or reverse aging [2,3].
Many functions of the skin
decrease with aging; among these are the renewal of cells, chemical cleansing,
mechanical protection, immune response, DNA repair, production of sweat and
sebum, and vitamin D production [3].This study aims to evaluate the
morphological changes associated with physiological aging in the skin of rats,
starting from the intrauterineperiod, and toprovide a preliminary foundation on
which further studies can build.
MATERIALS AND METHOD
This study was conducted on four different age
groups, each group consisting of eight rats, as follows: Group 1: intrauterine
(prenatal) day 19; group 2: postpartum (postnatal) day 21; group 3: postpartum
(postnatal) day 60; and group 4: postpartum (postnatal) month 19.
Under ketamine/xylazine anesthesia, skin
samples from the back, abdomen, head, and upper and lower limbs were obtained
from each subject. After routine tissue processing the sections were stained
with Mayer’s hematoxylin-eosin (H-E) for the evaluation of epidermal thickness,
Masson’s trichrome for dermal thickness, periodic acid-Schiff for basal
membrane thickness, elastic Van Gieson for the evaluation of elastic fibers,
toluidine blue for mast cell count, and Mowry’s colloidal iron for the
evaluation of glycosaminoglycans (GAGs). The stained specimens were examined
and photographed under a Leica DM LB2 microscope, and measurements were taken
using the Leica Q-Win Plus analytical system.
For all measurements, five different sections
were evaluated per specimen. For the determination of epidermal and dermal
thicknesses, under ×10 magnification, eight different areas from all five
sections were measured. Measurements of the number, height, and width of the
dermal papillae were made from five different areas under the × 10
magnification. For the basal membrane thickness, six different areas from all
five sections were measured under × 100 magnifications. Mast cell counts were
obtained from 15 different areas under × 100 magnifications and pilosebaceous
unit counts were obtained from five different areas under × 20 magnification.
STATISTICAL ANALYSIS
For the statistical analysis of all results, 13.0SPSS
for Windows software was used. All data are presented as the mean ± standard
deviation (SD). The Shapiro–Wilk normality test proved that our quantitative
variables did not exhibit a normal distribution (p < 0.05). Comparison of
variables in each group with respect to time variables was performed using the
Wilcoxon signed-rank test as a non-parametric test. Results were regarded as
statistically significant at p < 0.05.
RESULTS
The skin samples from the
back, abdomen, head, and upper and lower limbs obtained from the intrauterine
day 19 group showed a fairly regular stratified squamous epithelium rich in
cells. In the specimens obtained from the day 21 group, the epidermis showed a
four-layered structure, as observed in all other groups. The day 60 groupdid
not show any regional differences with respect to the epidermal layers, cell
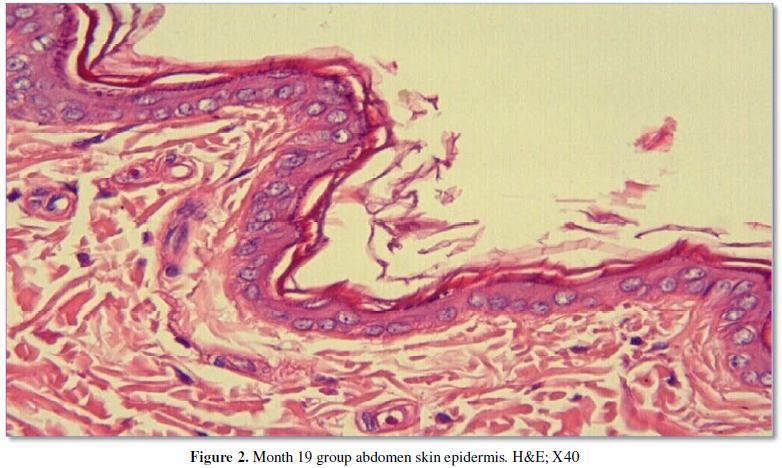
distribution, and morphology. Specimens from the month 19 group also showed two
or three rows of epithelial cells (Figure
1, 2). All measurement results are shown in the Table 1 and Graphic 1.
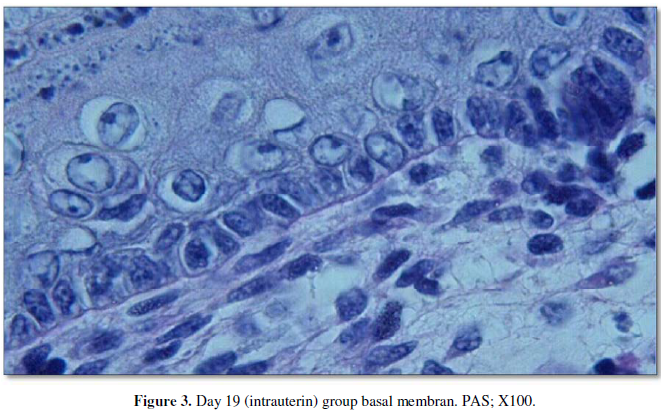
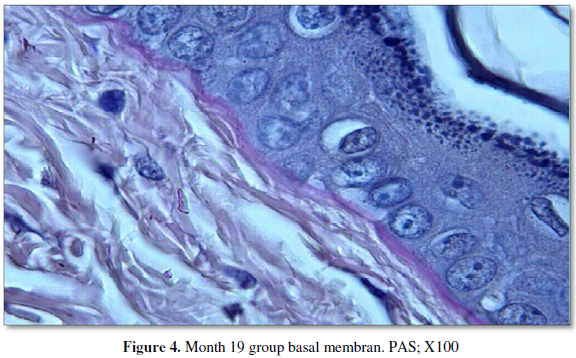
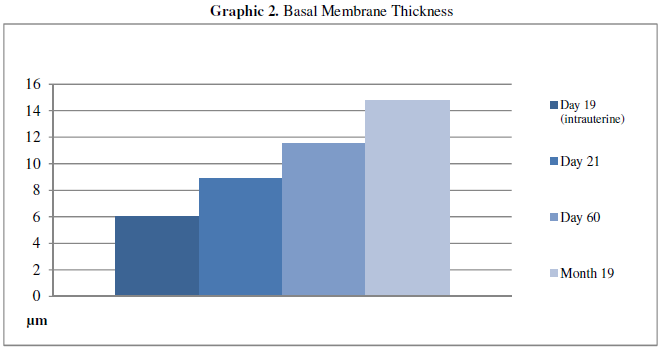
Examination of the Periodic Acid-Schiff stained specimens showed that the day
19 (intrauterine) group had the thinnest basal membrane, while the thickness
gradually increased in the day 21 and day 60 groups. However, the thickest basal
membrane was observed in the day 19 group (Figure
3, 4). All measurement results are shown in the Table 2 and Graphic 2.
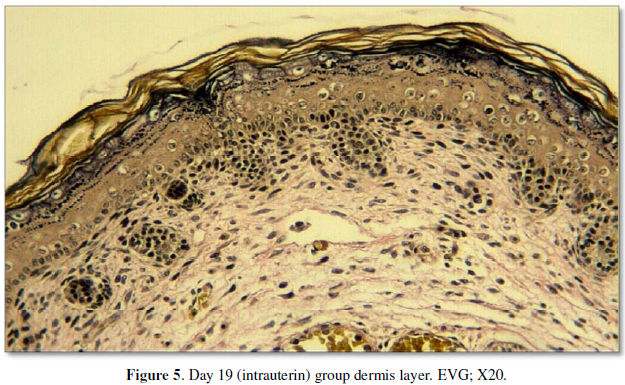
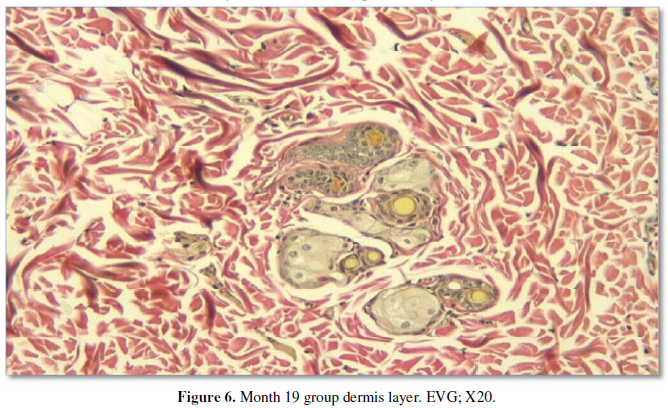
The dermis was examined using elastic Van
Gieson (EVG) and Masson’s trichrome stains. The prenatal group had the thinnest dermis and was relatively rich in
cells. Mowry’s colloidal iron-stained specimens obtained from this group showed
the highest level of GAGs. Inthepostpartumday 21 group, besides being rich in
cells, the fibrils were longer and had a greater diameter than those in the intrauterine
group. The hypodermis was also thicker and was easily distinguishable from the
overlying dermis. In general, in this group, elastic fibers were more prominent
than the intrauterine group and mostly condensed in the middle of the dermis.
In the postpartum day 60 group, the dermis appeared strikingly dense and was
heavily stained due to the abundance and thickness of fibrils. Furthermore,
among all the groups, the thickest dermis was observed in this group (Figure 5, 6). All measurement results
are shown in the Table 3 and Graphic 3.
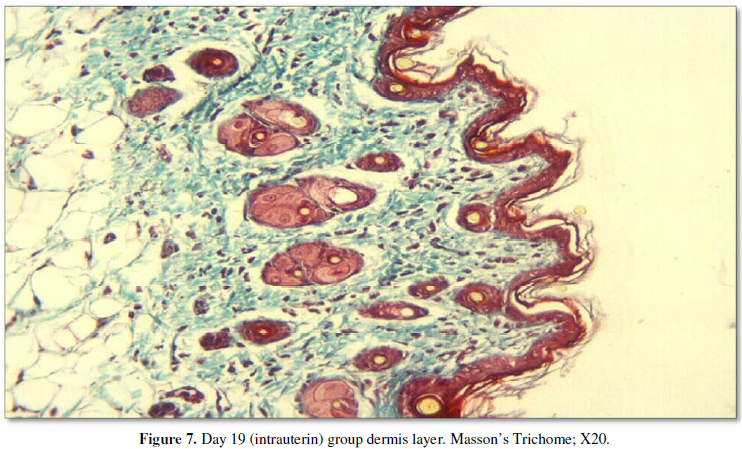
Observation of the back, abdomen, and head and
limb specimens did not show any major differences in the structure of the
dermis; the hypodermis was evident and was easily distinguishable from the
overlying dermis. Papillary dermis and reticular dermis were also clearly
distinguishable. Blue-stained acid mucopolysaccharides were generally present
around the pilosebaceous units, although they were also scattered within the
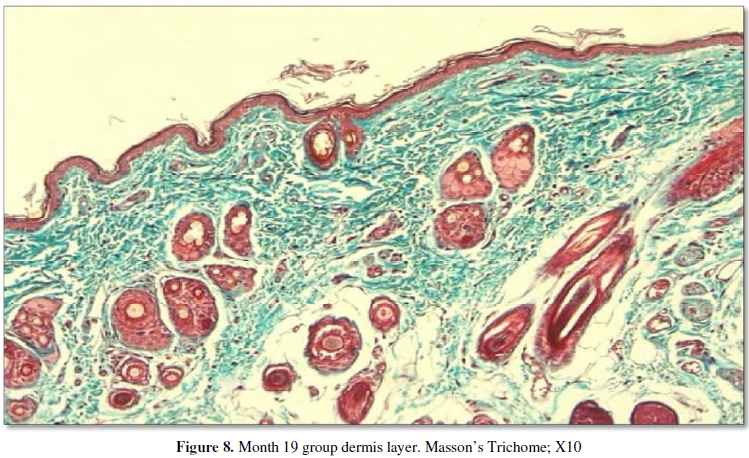
dermis. In the aged group, the hypodermis was quite evident, while the dermis
was more darkly stained and thinner than in the adult group although it
presented the same structure in both groups (Figure 7, 8). In the
prenatal group, the dermal papillae, which are projections of the dermis toward
the epidermis, were very few in number and also quite small in thickness and
height. All measurement results are shown in the Tables 4-6 and Graphics
4-6 Mast cell counts are shown in the Table 7 and Graphic 7,
and pilosebaceous units counts are shown in the Table 8 and Graphic
8.
CONCLUSION
To our knowledge, no previous morphological studies
evaluated the skin changes from the beginning of the intrauterine period by
considering parameters such as the thicknesses of the epidermis and dermis, the
numbers of pilosebaceous units and mast cells, and the structures of collagen,
elastic fibers, and GAGs of ratormouse.
In conclusion, the results of this study showed that the thicknesses of the epidermis, dermis, and basal lamina of rats change with aging. In addition, aging rats exhibit flattening at the dermoepidermal junction and changes in the composition of collagen, elastic fibers, and GAGs, finally resulting in quantitative changes in pilosebaceous units, vessels, and mast cells. We believe that these findings in rats might be helpful for understanding the skin changes during aging and may provide a foundation for further studies on skin aging.
- Norman RA, Henderson JN (2003) Aging: an overview. Dermatol Ther 16:
181-185.
- Baumann L (2007) Skin ageing its treatment. J Pathol 2112: 241-251.
- Scharffetter-Kochanek K (2001) Skin aging. Clin Exp Dermatol 26:
561-568.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of AIDS (ISSN: 2644-3023)