955
Views & Citations10
Likes & Shares
Platelet-Rich-Plasma (PRP) is an autologous
blood solution, rich in hundreds of growth factors and cytokines that during an
inactive state are found in platelet granules. There are a number of commercial
companies established that allow clinicians to synthesize and use PRP for
orthopedic, cosmetic, intramuscular injections (IM) and wound healing
procedures. The premise being that the increased growth factor concentration
expedites the desired treatment upon activation and granule exocytosis. PRP is
synthesized through a double centrifugation aseptic or sterile process. Robert
E. Marx, DDS is credited for being the pioneer of PRP use in oral and
maxillofacial surgical procedures and has even defined the working definition
of PRP to-date [1].
In this study PRP was synthesized using a
modified Messora and Nagata protocol [2]. Porcine acid citrate dextrose (ACD)
treated whole blood was purchased from Lampire Biological Laboratories and
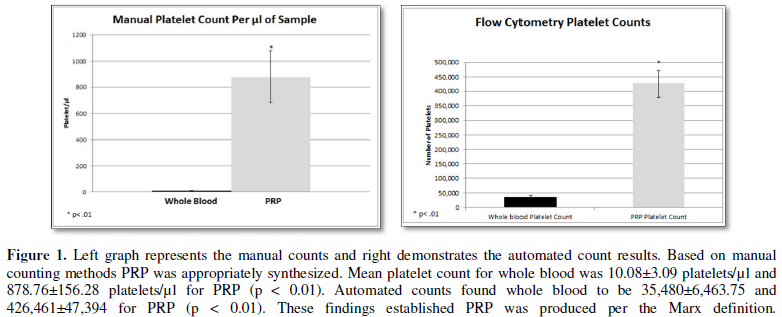
quantified both manually and via an automated flow cytometry method ensuring an
appropriate PRP concentration was produced (Figure 1). Based on manual counting methods PRP was appropriately
synthesized. Mean platelet count for whole blood was 10.08 ± 3.09 platelets/µl
and 878.76 ± 156.28 platelets/µl for PRP (p < 0.01). Automated counts found
whole blood to be 35,480 ± 6,463.75 and 426,461 ± 47,394 for PRP (p < 0.01).
These findings established PRP was produced per the Marx definition.
Following manual and automated verification visual
assessment was performed. Using light microscopy (LM) and scanning electron
microscopy (SEM) the PRP was determined to be in an inactive state and was
activated on-demand using an electrospun collagen scaffold that was synthesized
by the published in-house laboratory protocol [3]. Verification of activated
platelets was morphologically validated using prior established literature on
pseudopod, bleb formation and platelet clumping. Following morphological
verification of inactive and activated forms a bench-top wound healing assay
named the scratch assay was implemented to demonstrate the efficacy of the PRP
in a wound model prior to mammalian surgical studies. Figure 2 below
demonstrates the SEM morphologic evaluation.
Using the scratch assay allows for bench-top
validation prior to expensive mammalian studies to occur, this assay acts as a
proof of concept and has been validated and established as a useful test in the
pre-clinical literature [4]. Initial “scratches” measuring 1.5mm ± 0.5 mm were
created in seeded tissue culture confluent monolayers of human neo-natal dermal
fibroblast wells with growth media (stock Dulbecos Modified Eagle Medium). Each
well was measure for wound closure at four time points (0 4, 8, and 12 hours).
Each well was also given a specific concentration of PRP which was able to be
viewed using LM for closure assessment. Concentrations of PRP ranged from
0.25%, 0.125%, 0.063%, 0.031%, 0.016% and 0.008% (this experiment was repeated
multiple times to increase the sample size). These dilutions were selected as a
result of the inability for 100% PRP concentrations to be viewed through the
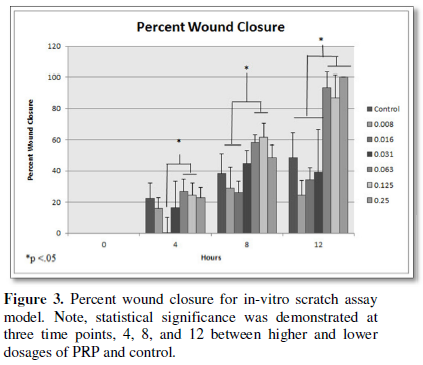
field of view in LM. Upon statistically assessment of percent wound closure in
the scratch assay the higher percentages of PRP closed the wounds at earlier
time points compared to lower dosages and the control. Figure 3 below
demonstrates the collective findings.
Once bench-top validation had been demonstrated
IACUC approval was sought out and granted from Northern Arizona University
(NAU) #12-006R1. Using four eight-week old female murine hairless SCID mice a
full-thickness wound study was implemented. SCID murine models were selected
due to the use of porcine whole blood to synthesize the PRP. The SCID model
will negated immunological complications from varying animal tissue sources.
Two treatments and a control were applied in the study. The treatments included
PRP with an electrospun collagen scaffold (combination therapy) used for
platelet activation, a standalone collagen scaffold and lastly standard wound
care treatment (sterile gauze and Coban wrap to prevent wound site
interference). Assessment of wound
sites was performed using photographic images at time points 0, 2, 4 and 6
days. The images were then analyzed with NIH Image J software for percent wound
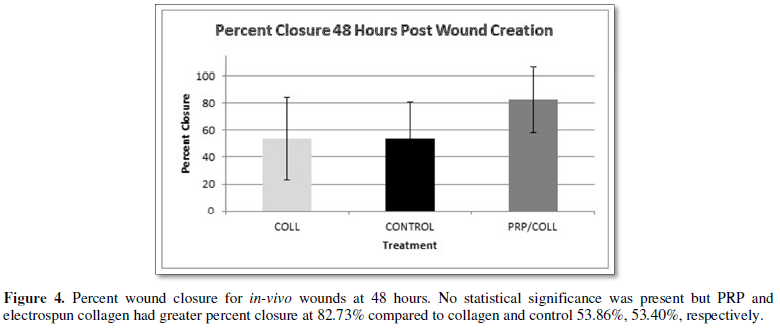
closure. Figures 4-6 detail the findings at the set time points.
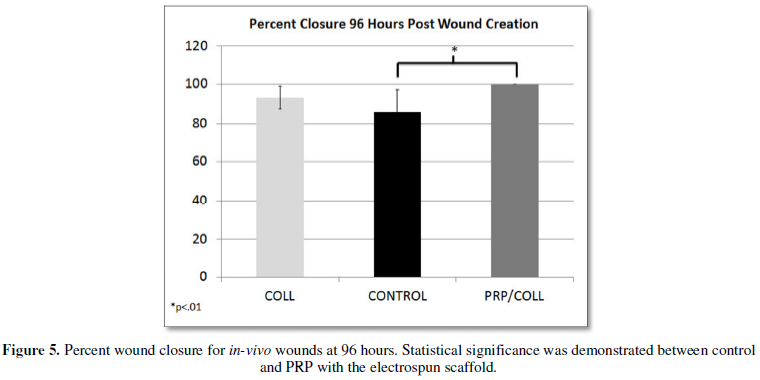
At 96 hours post wound creation the combination therapy, PRP with electrospun
collagen scaffolds, had a percent closure of 100% compared to the control at
86.076 ± 11.28% (p<0.01). At the
same time point, the standalone collagen treatment had a mean closure of 93.15
± 5.66% and did not demonstrate statistical significance compared to the
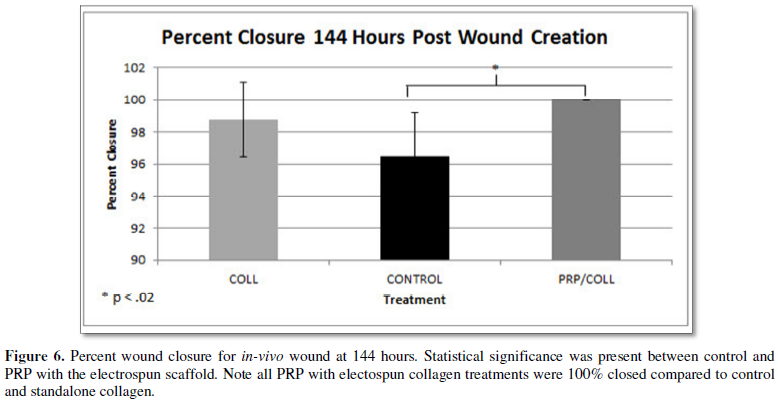
control (Figure 5). The same trends were seen at 144 hours post wound
creation with the combination treatment having a percent closure of 100% while
the control had a percent closure of 96.48 ± 2.724% (p<0.02). The standalone collagen treatment had a percent closure
of 98.741 ± 2.340% and again lacked statistical significance compared to the
control.
As based on the study results PRP was created, per the Marx
definition and PRP does aid in expediting the wound healing process by
increasing the percent wound closure within a specified study timeframe (144
hours total). Thiscan subsequently be transferred from a pre-clinical to a
human clinical relationship. Furthermore when PRP is combined with an
electrospun collagen scaffold activation of the platelets occurs releasing the
hundreds of growth factors and proteins into the wound bed. This supports the
pre-existing literature that PRP can aid in tissue regeneration as a result of
this growth factor concentrate. The ability to delivery extracellular membrane
scaffolds, such as the collagen scaffold also aids in the ability to an
expedited wound healing event. This happens due to the presence of the
extracellular membrane which would normally need to be deposited during the
matrix deposition phase of the wound healing cascade, here the membrane is
delivered aiding to reduce the potential time needed in that specific
deposition phase. In all PRP combined with a collagen scaffold does expedite
the wound healing event and can serve as another novel technique to clinically
treat full-thickness wounds. Furthermore it may serve as an alternative for
patients who may be opposed to traditional treatment modalities. For instance
those who may refuse drug intervention or others; the ability to use autologous
PRP may eliminate these patient concerns when a clinician is treating the
patient with their own body tissues.
Declarations
No competing financial interests exist in this study.
- Marx RE (2001)
Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent 10: 225-228.
- Messora MR,
Nagata MJH, Furlaneto FAC, Dornelles RCM, Bomfim SRM, et al. (2011) A
standardized research protocol for platelet-rich plasma (PRP) preparation
in rats. RSBO 8: 299-304.
- Machula H,
Ensley B, Kellar R (2014) Electrospuntropo elastin for delivery of
therapeutic adipose-derived stem cells to full-thickness dermal wounds. Adv Wound Care 3: 367-375.
- Yarrow JC,
Perlman ZE, Westwood NJ, Mitchison TJ (2004) A high-throughput cell
migration assay using scratch wound healing, a comparison of image-based
readout methods. BMC Biotechnol
4.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Spine Diseases
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)